27 Aug 2018
Emi Barker looks at how symptoms arise, investigation methods and approaches to treatment.

For the purpose of this article, urinary tract infection (UTI) will be defined as bacterial infection of the bladder, which comprises the overwhelming majority of UTIs.
Less frequently, bacterial infections may also involve the kidneys (for example, pyelonephritis or pyonephritis), ureters, urethra or components of the genital tract associated with the urinary system (for example, prostatitis, orchitis or vulvulovaginitis) – these will not be specifically discussed in this article. Rarely, infections with other pathogenic organisms (such as fungi or nematodes) may occur.
Key learning points for UTIs are to:
Dysuria: abnormal passage of urine.
Stranguria: painful and difficult passage of urine.
Haematuria: blood in the urine.
Pollakiuria: increased frequency in urination, typically not associated with an increase in overall daily urine volume.
Periuria: urination in inappropriate places – for example, in the pet’s bed or kitchen near to an external door.
Incontinence: loss of voluntary control over urination, although owners may be unable to determine whether their pet has control over its urination – for example, unwitnessed urination indoors or may present as urination on picking up the pet.
Malodorous urine.
Systemic signs*:
* The presence of systemic signs should raise suspicion of a complicated urinary tract infection – such as deep-tissue infection, infection involving the kidneys or prostate, or the presence of concurrent disease.
Clinical signs consistent with UTIs (Panel 1) are a common reason for presentation to primary vets, with one in seven dogs being diagnosed with a UTI during the course of its lifetime1.
However, the presence or absence of these clinical signs cannot be used to confirm or exclude the presence of a UTI. In a study, only half of dogs presenting with signs consistent with UTIs were confirmed to have the condition2.
In contrast, dogs may have significant bacteriuria in the absence of clinical signs suggestive of a UTI3, such as those that are diabetic or have hyperadrenocorticism. To complicate matters further, studies have also found a small percentage of dogs (2% to 9%) to have transient or persistent subclinical bacteriuria (that is, positive urine culture in the absence of clinical signs in otherwise healthy patients) that do not require antimicrobial treatment4,5.
The clinical signs associated with lower urinary tract disease are not specific for UTIs. Other differentials that may occur concurrently include:
As indicated earlier, the majority of uncomplicated UTIs are due to bacteria. Of these, most infections involve opportunistic pathogens ascending from the perineal region into the urinary bladder. Rarely, UTIs result from other routes such as haematogenous spread. A number of host mechanisms act to resist the ascension of these organisms. The development of UTIs, therefore, occurs when bacteria overcome these host barriers as a result of a loss of host-resistance mechanisms (Panel 2), or the presence of bacterial virulence factors, or a combination of the two.
Failure to address any contributory host risk factors, as part of the management of the UTI, can lead to persistent, recurrent or relapsing infection. These risk factors may be multifactorial and change over time. For example, an obese female dog with urinary incontinence following ovariohysterectomy may develop a Proteus species UTI, which, in turn, may result in the formation of struvite urolithiasis. As well as the presence of a potentially invasive uropathogen (Proteus), this dog is obese, has USMI and uroliths – all of which will need addressing concurrently.
Structural deficits:
Loss of urethral sphincter function:
Incomplete bladder voiding:
Loss of urine bacteriostatic/bacteriocidal properties (for example, alterations in pH – although these may also be seen secondary to UTIs):
Lack of host response:
Agents of bacterial UTIs are opportunistic pathogens derived from either faecal flora (such as Escherichia coli – accounting for approximately half of all isolates – Proteus mirabilis, Enterococcus species and Klebsiella species) or skin commensals (predominantly Streptococcus species and Staphylococcus species)6,7.
More recently, closer examination of UTI bacterial isolates has revealed some with a particular tropism for the urinary tract – for example, uropathogenic E coli or Corynebacterium urealyticum3,7. Some of these agents have virulence factors that allow them to invade the deeper tissues of the urinary tract or form biofilms – resulting in relative protection from antibiotics concentrated within the urine, leading to persistent or relapsing infection.
It should be noted some agents (for example, Enterococcus species and C urealyticum) are intrinsically resistant to some antimicrobial agents, yet they may also acquire resistance to other antimicrobial agents. Some intrinsic in vivo resistance patterns may not reflect their in vitro laboratory-derived resistance pattern – for example, Enterococcus species are intrinsically resistant to trimethoprim and sulfonamide as they can obtain folate from their host, whereas this nutrient is not available in standard growth media, resulting in apparent susceptibility to trimethoprim and sulfonamide in the laboratory (note trimethoprim and sulfonamide interferes with bacterial folate metabolism).
History, physical examination findings and basic urinalysis (dipstick and sediment examination) may support a diagnosis of UTI. Owners may be requested to collect a urine sample from their pet prior to the consultation. Devices are available (Figure 1) to facilitate the free-catch collection of urine.
A thorough clinical history is required to confirm the nature of the owner’s main complaint (for example, differentiating pollakiuria from polyuria, incontinence from periuria), determining whether this is a recurrent issue and establishing whether any known predisposing factors exist (for example, concurrent disease or medications).
A complete physical examination, including rectal palpation in both male and female dogs, is required to assess for any evidence of concurrent disease and/or systemic involvement. Abdominal palpation may reveal pain, but also indicate the size of the bladder, which is useful when planning further investigation. Basic urinalysis (on free-catch or cystocentesis-obtained sample) is then performed to support clinical suspicion.
Where evidence of systemic disease (for example, weight loss, pyrexia, polyuria/polydipsia, mentation changes) or an underlying complicating factor exist (such as ectopic ureters or urolithiasis) – or, in the case of persistent, relapsing or recurrent UTIs – further investigations are warranted. These include haematology and serum biochemistry, plus appropriate imaging of the urinary tract.
Visible assessment findings of urine should be noted. Marked pigmenturia (including haematuria) can result in false positive results for some of the dipstick tests. Turbidity may be increased with UTI, due to pyuria, haematuria, mucus and, potentially, (struvite) crystal formation; however, this is neither sensitive nor specific. Some individuals may also detect the odour of ketones in urine, which raises the suspicion of unstable diabetes mellitus – a risk factor for UTI. An increased smell of ammonia may be detected with the presence of UTIs, or as a storage artefact.
It should be noted that, unlike in cats, urine concentration in dogs can be very variable in the normal state. However, this may be useful in excluding polyuria/polydipsia as a contributory factor, and it can further characterise the nature of azotaemia if noted on serum biochemistry.
The presence of alkaline in the urine (pH greater than seven) may support the presence of a urea-splitting bacteria, such as Staphylococcus species, Proteus species or Klebsiella species; however, it is not specific for UTI.
Urine protein concentrations (UPC) may be increased in the presence of UTI, due to host inflammatory response, resulting in positive results on urine dipstick or urine protein to creatinine ratio measurement (if measured). As a result, interpretation of UPC as a marker of renal glomerular protein loss is limited in the presence of a UTI.

Dipstick analysis may identify risk factors for the development of UTIs (such as glycosuria or ketonuria). The presence of haematuria also supports a clinical suspicion of UTI (in conjunction with other findings), but is a non-specific finding. The presence of a positive leukocyte result is supportive of a UTI in dogs (note, not in cats), but very poorly sensitive.
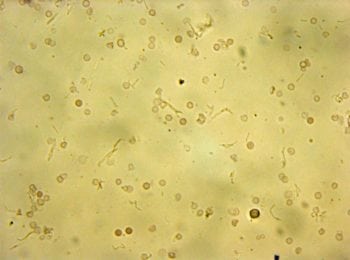
Although very low numbers of red and white blood cells may be noted in the urine of normal dogs (particularly if collected by cystocentesis), where marked increases in their numbers exist, this supports the suspicion of UTI (Figure 2a). Other differentials include neoplasia and urolithiasis. Bacteria may also be seen in conjunction with the pyuria.
When bacteria are noted intracellularly, this is consistent with active infection. However, the absence of bacteria does not rule out UTI (for example, if present only at low levels or if antibiotics were recently administered), bacteria may be seen in the absence of pyuria (such as subclinical bacteriuria, immunocompromised host, contamination and storage artefact) and if the sample analysis is free-catch collected, this could represent infection distal to the bladder. The presence of casts raises the suspicion of renal involvement, although small numbers of hyaline or granular casts may be present in the urine of normal dogs (particularly if well concentrated).
Crystals may be noted on sediment examination. Their significance depends on their type, urine biochemical factors, and time and storage from collection to analysis.
Some crystals may occur as a result of storage artefact – particularly if cooled/refrigerated with a high urine specific gravity. Where the presence of crystalluria raises concern regarding urolithiasis, urinary tract imaging (for example, ultrasound or contrast radiography) is indicated. Further testing, for example, for genetic mutations or hepatic dysfunction, may also be considered as appropriate.
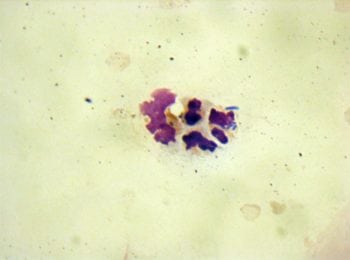
In addition to sediment examination, urine may also be submitted for cytology (Figure 2b). For this, urine (typically 0.5ml to 1ml) is placed in a ethylenediamine tetra-acetic acid blood collection tube to preserve cell morphology.
Cytospin preparations of urine, using a modified Wright stain, are viewed under oil immersion. This is frequently performed at the author’s institute as it appears to provide a more sensitive and specific evaluation of bacteriuria, and host response, than sediment examination alone. It may also indicate the nature of the bacterial infection (such as coccoid, bacilli or mixed) to facilitate early treatment recommendations pending urine culture results, or the presence of neoplastic cells where concurrent disease is present.
Where severe infection is present, dysplastic cells may be detected in the urine. These dysplastic cells can share features with neoplastic urothelial cells. Repeat testing and imaging should be considered in these cases.
Where UTI is suspected, a urine sample (collected by cystocentesis) should be submitted for anaerobic culture prior to treatment, to confirm clinical suspicion before therapy is instigated. Urine collection by sterile urinary catheterisation (for example, from a male dog) is less ideal and, in the author’s experience, more time consuming.
Urine culture from free-catch samples is not recommended; although a negative result may rule out UTI, a positive result should be confirmed by analysis of a cystocentesis sample (this often increases the cost to the client, and either delays treatment or is of limited benefit if antimicrobial treatment has already been given pending results).
Antibiotic sensitivity should be determined for cultured isolates. Note, separate tubes should be used for urine culture and urinalysis (to prevent contamination), and where a delay in submission to the laboratory will occur, use of a boric acid tube should be considered.
If urine culture is positive, no indication exists for bladder wall biopsies to obtain samples for culture8, although tissue biopsy of histopathology may be indicated if neoplasia is strongly suspected. If surgery is necessary (for example, to remove uroliths) a bladder wall sample could be submitted for culture, along with a representative sample of the uroliths obtained. Uroliths should also be submitted for composition analysis as crystalluria may only reflect their underlying pathology.
The International Society for Companion Animal Infectious Diseases has produced consensus guidelines on the diagnosis and treatment of canine UTIs9. This is an open access online article and is worth reviewing, particularly when formulating local practice guidelines. This article does not discuss the additional management considerations of patients that have obstructed urinary tracts. Even those with partial obstruction – for example, as a result of tissue inflammation – warrant close monitoring.
Otherwise healthy female dogs – with no co-morbidities or history of recurrent infections – can be considered uncomplicated. Uncomplicated UTIs can be managed with antimicrobial agents, pending culture results, to relieve clinical signs.
Suggested first-line agents include amoxicillin or trimethoprim and sulfonamide; however, with limited availability of some of these agents, either due to local practice pharmacy stocking or withdrawal from the veterinary market, amoxicillin and clavulanate is considered an acceptable alternative.
Penicillins are actively excreted into urine, obtaining much higher concentrations than those found in tissues, therefore they are particularly effective in the management of most UTIs (in the absence of resistance). The manufacturer’s recommendations for amoxicillin and clavulanate administration is 12.5mg/kg orally twice daily for 5 to 7 days; however, it should be noted the consensus statement9 suggests 12.5mg/kg to 25mg/kg orally three times daily for 7 to 14 days.
Where clinical signs are suggestive of systemic involvement (for example, polyuria/polydipsia or mentation changes) or where abdominal imaging has demonstrated severe or irregular thickening of the bladder wall, the author considers three times daily administration at these higher doses. The author has managed cases where twice daily administration of amoxicillin and clavulanate had failed to resolve UTIs – presumably due to tissue invasion by bacteria – whereas increased dose frequency to three times daily resulted in the clearance of infection.
If culture results are negative, and a false negative result is not suspected, antimicrobial treatment can be discontinued. If clinical signs are persistent, further investigation of alternative diagnoses are warranted. If culture results indicate sensitivity to the drug administered, but clinical signs are persistent, further investigation of concurrent urinary tract disorders (such as urolithiasis) are warranted. If culture results indicate resistance to the drug administered, but clinical signs have resolved, the treatment course should be completed and the dog closely monitored.
If culture results indicate resistance to the drug administered, but clinical signs are persistent, an alternative antimicrobial treatment should be chosen based on the sensitivity pattern. Follow-up urine culture needs not be performed in these cases if clinical signs are resolved.
Dogs with co-morbidities, structural deficits within the urinary tract, or recurrent/refractory disease can be considered complicated. Male dogs may also be considered as complicated as the anatomy of their urinary tract reduces the risk of UTIs. In addition to antimicrobial treatment, investigation and concurrent management of underlying contributory factors or co-morbidities is indicated. Where the UTI has relapsed, and particularly if refractory to treatment, owner factors, such as compliance, should also be explored. Recurrent or refractory cases may require referral to a medical specialist.
Descriptions for complicated UTIs are:
Ideally, drug choice would be based on culture and sensitivity testing. However, if necessary, these dogs can be managed with antimicrobial agents pending culture results to relieve clinical signs. Again, first-line agents include amoxicillin and clavulanate, or trimethoprim and sulfonamide. If culture results indicate resistance to the drug administered, an alternative antimicrobial treatment should be chosen based on the sensitivity pattern.
In entire male dogs, administration of treatment that provides good coverage of assumed concurrent prostatic infection is recommended. As the blood-prostate barrier may limit bacterial penetrations, drugs, such as the fluoroquinolone enrofloxacin, or trimethoprim and sulfonamide, have been previously suggested. However, in more acute disease, when the patient is often systemically unwell, this blood-prostate is likely disrupted and alternative antibiotics (administered to provide tissue coverage) can be considered.
As prostatic hyperplasia commonly contributed to the persistence of infection, chemical castration should be considered pending stabilisation, with subsequent surgical castration discussed with the client.
For catheter-associated UTIs, ideally, the device should be removed. Its presence provides a surface on which biofilms can develop. Furthermore, if antimicrobial treatment is administered while they remain in place, the risk of drug resistance increases.
Although little evidence base exists to support this, treatment durations of four weeks have been suggested, but this should be based on history, clinical findings and reason for complication. Urine culture is ideally performed while receiving treatment (for example, after seven days), and then again one week following cessation of treatment. If the UTI recurs, further investigation, if not already performed, is warranted.
Analgesia, particularly NSAIDs, can be considered to relieve some of the clinical signs associated with pain. These should only be required for a few days, pending response to antimicrobial agents.
Little evidence exists to support the use of glucosamine supplements or cranberry extracts in humans, dogs or cats with urinary tract disease. One study showed no difference in the frequency of bacteriuria in dogs with thoracolumbar disc disease when given cranberry extract10. The use of glucosamines in the management of cats with idiopathic cystitis also found no benefit11.
In human medicine, evidence is increasing that probiotic administration may benefit those with chronic and recurrent UTIs. The use of gastrointestinal probiotics in the management of UTIs in dogs has yet to be evaluated; however, given the theoretical benefit of stabilising the gastrointestinal flora of patients with chronic UTIs – particularly of those receiving extended courses of antibiotics – their administration has the potential to be of benefit and is not considered harmful.
Encouraging the patient to frequently empty its bladder by offering opportunities to do so (such as multiple walks or unlimited access to outdoors), as well as increasing voluntary water intake (for example, feeding a wet diet with increased water content) will be of benefit. Diet may also be used to reduce crystal formation and modify pH.
Although UTIs are very common in our canine patients, we suspect their presence to be more common than they actually occur. In light of increased concern regarding over-prescription of antimicrobial agents, and the promotion of responsible antimicrobial stewardship, we should consider differential diagnoses in these patients and justify our use of even the more routinely used agents. In particular, where recurrent infections occur, or co-morbidities exist, we should aim to treat these patients holistically.