27 Feb 2017
Mateusz Jaksz and Claudia Busse, in the first of a two-part article, look at aetiology and clinical signs for this common eye issue, paying particular attention to superficial ulcers.

Corneal ulcers are one of the most common problems of the canine eye seen in general veterinary practice. The term “corneal ulcer” describes the loss of continuity of the corneal epithelium with exposure of the underlying stroma and covers a range of lesions that can be classified according to depth and extent.
Most corneal ulcers result from a variety of traumatic causes and usually respond well to medical therapy alone. However, some may be very deep or extensive, become infected, heal poorly or deteriorate, despite medical treatment. This article aims to give an overview of the different types of corneal ulcerations and treatment modalities for uncomplicated and non-healing superficial ulcers. Part two will address the diagnosis and treatment of deeper corneal defects.
The cornea is the transparent anterior wall of the eye that refracts light on to the retina. It contributes to the main part of the eye‘s total optical power and, to achieve optimal vision, must remain clear.
Corneal transparency is maintained by its highly organised structure, relative dehydration, and lack of vessels and pigment.
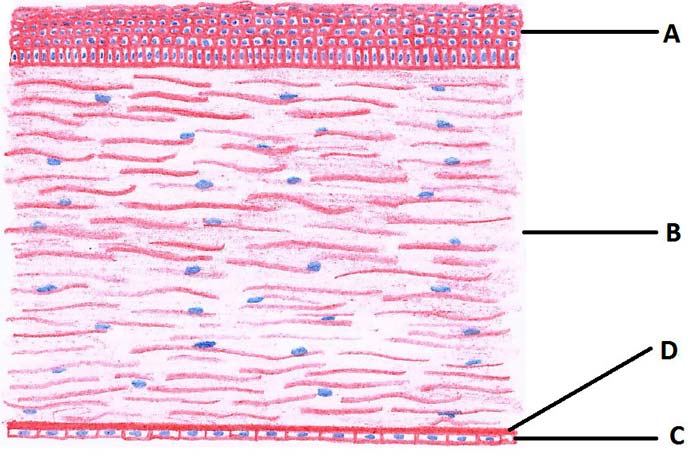
In dogs, the cornea is about 0.5mm to 0.6mm thick and its central area is slightly thinner than the periphery. The cornea consists of three layers that, from outside to inside, are:
The corneal epithelium is a multicellular layer of non-keratinised stratified squamous epithelial cells that forms about 10% of the corneal thickness. It is attached to the underlying stroma through a number of adhesion complexes and undergoes a constant cycle of shedding and basal cell proliferation. The stroma comprises 90% of the corneal thickness and provides its main mechanical strength. It is formed of collagen fibres that are arranged in parallel layers and keratocytes sparsely scattered between them. They are surrounded by ground substance, composed of proteoglycans, which help maintain corneal hydration and transparency.
The most inner corneal layer is the endothelium with its basement membrane, Descemet’s membrane. The endothelium is a single layer of cells and its main function is the dehydration of the cornea.
Each corneal layer responds to injury in a different way. Moreover, many factors influence the process of healing, such as breed, age, tear film quality, palpebral conformation, blink rate or concomitant metabolic disorders.
Epithelial cells migrate and multiply to cover any defect at a rate of roughly 1mm per day. After 24 hours of delay, the stem cells for the corneal epithelium divide to re-establish epithelial thickness. They travel in a centripetal pattern from the limbus. In dogs, complete restoration of the epithelium takes approximately 7 to 10 days. Uncomplicated superficial ulcers heal without associated fibrosis and scar formation within that time.

Stromal healing is a much slower process that involves transformation of keratocytes, production of collagen fibres and tissue remodeling. In very young animals, stromal healing can restore full initial corneal thickness, while, in adult or elderly animals, the cornea may remain thinner. Stromal injuries will always result in some degree of scarring. This is mainly because of transformation of normal keratocytes into fibroblasts. New collagen fibres and lamellae are produced in a less organised fashion, which leads to permanent opacification. More extensive and complicated ulcers stimulate corneal vascularisation – the area is invaded by vessels originating from the limbus. Granulation tissue may be formed, usually resulting in a denser scar.
Healing of the endothelium is limited, since these cells have minimal regenerative capabilities in dogs. They cover defects by hypertrophy and sliding, rather than mitosis, and this ability is limited. Damage to the endothelium disrupts the process that moves fluid out of the stroma, resulting in corneal oedema and loss of transparency in the area of the defect. This may be permanent if not enough endothelial cells remain to maintain the relative dehydration of the cornea.
Many external and internal factors can influence these healing mechanisms and complicate corneal defects. Therefore, it is essential to find the cause of corneal damage to establish optimal treatment.
It is always necessary to identify – and, if possible, eliminate – the underlying cause and any predisposing factors that may lead to corneal ulceration. Common causes include:
Animals with corneal ulcers present with signs of ocular pain, including blepharospasm, enophthalmos, protrusion of the third eyelid, miosis, conjunctival hyperaemia and excessive tearing. These signs vary depending on the depth of the ulcer; superficial ulcers may be more painful than deep ulcers due to the distribution of sensory nerve fibres.
Stimulation of corneal nerve endings triggers a trigeminal axonal reflex causing a so-called reflex uveitis, which significantly contributes to the discomfort seen in patients with corneal ulcers. Signs include miosis, a decreased intraocular pressure and aqueous humour flare.
The ophthalmic examination should include an initial gross hands-off examination to evaluate the general behaviour of the patient, symmetry of the face and eyes, as well as presence and type of ocular discharge.
The first part of the hands-on examination should be the Schirmer tear test (STT) to evaluate tear production as this may be influenced by further tests.
Tear production in an ulcerated eye is expected to exceed the normal range (15mm/minute to 25mm/minute). Values below 15mm/minute may be indicative of a dry eye syndrome.
The STT should be avoided in deep corneal defects or perforations, but may be performed in the contralateral eye instead for evidence of dry eye (keratoconjunctivitis sicca), as this is often a bilateral condition. Assessment of the tear production at a later date, when the eye has been stabilised, is recommended in deep defects. Corneal sampling for bacteriology and cytology should be carried out before the application of fluorescein.
A close-up examination of the eye with a focal light source is used to examine the extraocular structures and localise a possible source of corneal irritation. The thorough examination of the corneal surface – ideally with a slit lamp – enables the examiner to gauge the depth of an ulcer. In the absence of a slit lamp, this can be challenging. However, remembering the normal corneal thickness is only 0.5mm to 0.6mm, any indentation of the corneal surface should be considered as a significant defect with reduced corneal stability. The gold standard for assessing the true depth of a corneal defect remains the cross-sectional slit lamp view (Figure 2).
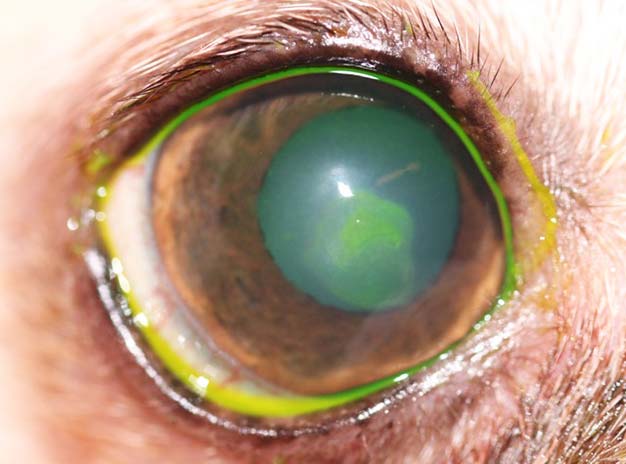
If the examiner is still unsure whether a corneal ulcer is present or how extensive it may be, the last diagnostic procedure may be the application of fluorescein dye. In a healthy cornea, the hydrophobic corneal epithelium prevents the distribution of the fluorescein solution within the stroma (Figure 3). Once this layer is damaged, the dye adheres to the hydrophilic stroma outlining the extent of the epithelial defect.
Descemet’s membrane does not absorb the stain; therefore, total stromal thickness loss appears as a dark, clear centre with a green boundary. However, if a deep defect with a clear centre is observed, the use of fluorescein should be avoided as the eye is very fragile and any manipulation may lead to a rupture of the ulcer.
Corneal ulcers are classified according to their depth, depending on the layers they affect. If only the epithelium is lost then they are classified as superficial ulcers. If the defect reaches into the stroma, it is called a deep or stromal ulcer.
Uncomplicated superficial ulcers involve the loss of the epithelial layer. This leads to a risk of secondary bacterial infection by the residential flora; therefore, topical broad-spectrum antibiotics should be used as routine therapy. Superficial ulcers can cause considerable pain and application of a mydriatic agent (atropine 1%) to relieve the painful ciliary muscle spasm should be considered if a reflex uveitis is present. Other pain relief options include the use of systemic NSAIDs and, if required, opioids.
If the underlying cause has been eliminated and corneal healing mechanisms are working properly, superficial ulcers should resolve within 7 to 10 days, at the most, depending on the size of the defect.
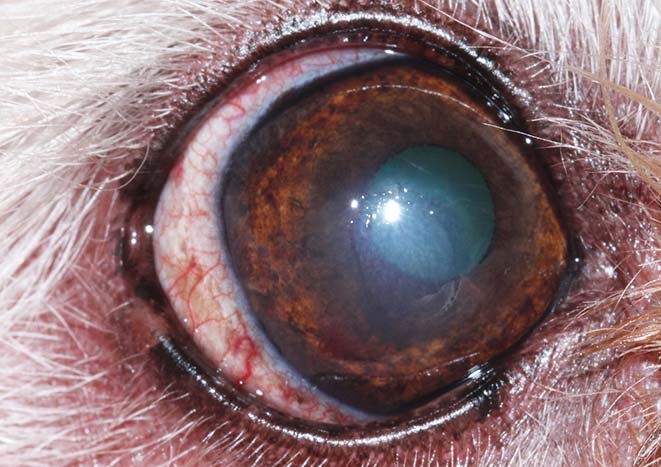
When the corneal wound-healing process does not progress as expected, the patient should, again, be evaluated for potential underlying or contributing problems, but if none are found following a detailed examination, a non-healing ulcer should be considered. Diagnostic criteria include the presence of an ulcer for more than two weeks, exclusion of all potential underlying factors that may delay healing and a characteristic appearance of the ulcer with loose epithelial edges, but without apparent involvement of the stroma (Figure 4).
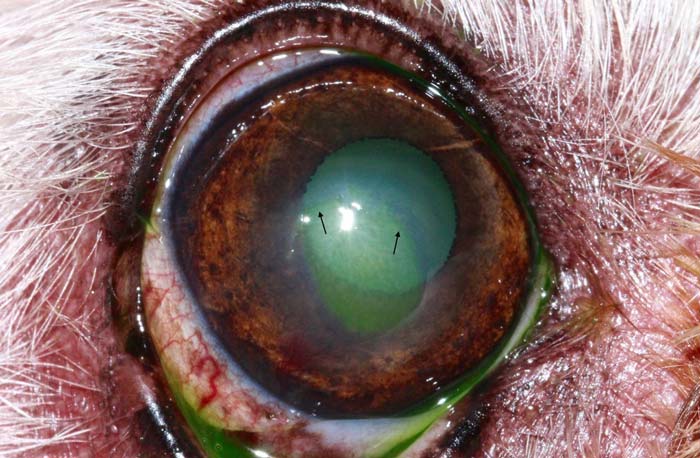
In the ophthalmic literature, this condition is referred to as spontaneous chronic corneal epithelial defect (SCCED), but other terms – such as boxer ulcer, refractory ulcer or indolent ulcer – are still commonly used. A SCCED is most likely triggered by a superficial corneal trauma, but the exact pathomechanism is not fully understood. A failure of attachment exists between the basal epithelium to its basement membrane and stroma. The presence of a hyaline acellular zone in the superficial stroma appears to be the main factor that prevents adhesion of the epithelium. The non-attachment results in a loose flap of epithelium under which fluorescein will migrate and be retained (Figure 5).
The ulcer persists – often failing to improve, but seldom worsening – for weeks to months. SCCED is reported mostly in middle-aged dogs, with boxers being overrepresented. Once the diagnosis of a non-healing ulcer has been made, it is important to educate the owner healing may take weeks to months and, sometimes, multiple treatments are required to resolve these lesions.
The ideal approach when treating non-healing ulcers should cover three main points: prevention of a secondary bacterial infection, pain management and encouraging healing. Prophylactic topical broad-spectrum antibiotics – such as fusidic acid, chloramphenicol or a triple antibiotic combination (neomycin, polymyxin B and bacitracin) two to four times daily – are used to prevent bacterial infection.
Pain relief is provided with cycloplegic drugs to relax the ciliary muscle spasm (for example, atropine eye drops to effect) and with systemic NSAIDs. The use of topical steroids or NSAIDs is contraindicated as they may delay healing. The latter have also been associated with initiating a melting process in human corneas.
The most common procedure to encourage healing is debridement of the corneal defect in conjunction with a keratotomy. Debridement is the removal of redundant epithelium and is performed by using clean – ideally sterile – cotton buds or ophthalmic spears. After application of a local anaesthetic, the loose epithelium is gently rubbed off following the direction of the ulcer margin. Normal “healthy” epithelium cannot be easily removed, so the procedure should be continued until only firmly adhered epithelium remains. The procedure can be repeated in 14-day intervals.
To encourage healing, debridement is usually combined with a superficial keratotomy either as punctuate, grid or diamond burr keratotomy. These procedures are performed after applying a topical anaesthetic and, if necessary, under sedation of the patient.
A grid keratotomy consists of parallel linear incisions through the very superficial corneal stroma in a grid-like fashion. It is performed with a 25-gauge to 27-gauge needle. The tip of the needle is used to gently draw linear incisions across the defect. These should be 1mm to 2mm apart and should extend 3mm into the normal cornea surrounding the ulcer. Care must be taken to avoid deep cuts that may result in gaping of corneal tissue.
Using a diamond burr (Figure 6) for this procedure is becoming more popular in veterinary medicine. Studies have shown a diamond burr treatment can significantly reduce the thickness or completely remove the hyaline acellular zone. This improves healing rates and results in less scarring of the treated cornea compared to more traditional treatment methods, such as a grid keratotomy.
Debridement and keratotomy can be repeated depending on the progress of the healing ulcer, but at least 10 to 14 days should be left between treatments to give the cornea time to heal. The examiner should remember to always check for missed underlying causes and consider a referral if healing is not achieved after one or, at most, two treatment attempts. Debridement and keratotomy are not an appropriate treatment for stromal defects in which one should always assume infection or an ongoing irritation, rather than a failure of the epithelium to connect with the underlying stroma.
A superficial keratectomy carries the highest success rate (close to 100%) with healing in 7 to 10 days, but is technically more demanding and requires a general anaesthesia and an operating microscope, and is more invasive for the cornea, resulting in a reduced corneal thickness and potentially more scarring. The very superficial corneal stroma in the affected area is removed. That completely removes the hyaline membrane and allows the renewal of normal epithelial adhesions.
In all named procedures, the placement of a soft bandage lens may provide mechanical protection to the healing cornea from trauma and eyelid rubbing. Postoperative care includes a topical antibiotic, a cycloplegic agent to effect and systemic NSAIDs. The animal should be re-evaluated 7 to 10 days after surgery to assess the progress of re-epithelialisation.
Various other therapies have been described for managing non-healing ulcers – these include:
None have been as safe and successful in treating non-healing ulcers as the debridement combined with a keratotomy.
Potential complications of non-healing ulcers include a secondary bacterial infection or a sterile corneal melt and excessive granulation tissue formation (Figure 7). Bacterial infection and sterile melt result in infiltration and enzymatic destruction of the stromal tissue. These complicated ulcers will be discussed in the second part of this article.
Formation of exuberant granulation tissue is often the result of excessive or repeated debridement. A fluorescein test should be performed to check if the corneal ulcer has healed. No treatment is required when the cornea is fluorescein negative. Nevertheless, if the ulceration is still present, the vascular change in the stroma may prevent adhesion of the epithelium.
Topical ciclosporin ointment can help reduce corneal vascularisation and, therefore, encourage adhesion between epithelium and stroma. Severe corneal vascularisation indicates significant scarring of the cornea is likely to occur. The process of regaining corneal transparency may take months, but improves with time. Again, ciclosporin ointment can encourage regression of corneal vascularisation or reduce possible corneal pigmentation.