27 Aug 2012
Alberta De Stefani-Llabrés and Anita Theobald discuss approaches to diagnosing senile dementia, and consider the various treatment protocols that can be applied to different cases.

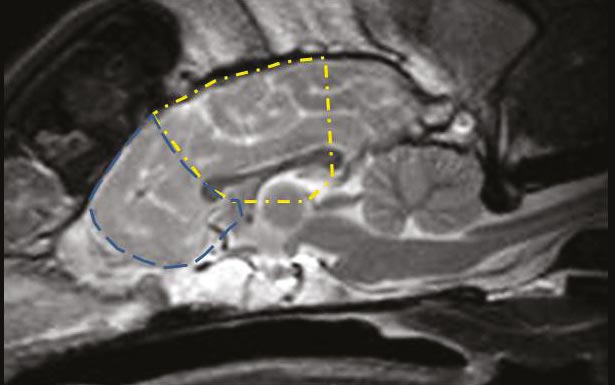
Figure 1. A midline sagittal T2-weighted MRI scan of a normal canine brain. The approximate location of the frontal lobe is outlined by the blue dashed line, while the approximate location of the parietal lobe (also commonly affected by age-related deterioration) is outlined in yellow.
Cognitive dysfunction syndrome (CDS) is the term used to describe the behavioural changes, and learning and memory impairment seen secondary to age-related degeneration of the brain.
Typically, affected dogs and cats present with a history of loss of orientation or apparent confusion in a familiar environment, loss of house training, abnormal social interactions and a change in sleeping patterns.
Despite potentially resulting in dramatic behavioural changes in pets – and significantly affecting the quality of life of the animal and owners – it has been reported canine cognitive dysfunction is likely to be underdiagnosed in the general canine population. Only 1.9% of a surveyed pet canine population (aged 8 years, to 19 years and 8 months) were reported to have been clinically diagnosed with the condition, despite an owner questionnaire revealing a further 14.2% displayed consistent behavioural changes1.
Signs of behavioural dysfunction also progress with time, with one study identifying further changes in behaviour detected 6 months to 18 months after the initial owner interview2.
The prevalence of behavioural change consistent with CDS in cats is also considered to be high. A study reported an owner questionnaire revealed 36% of cats aged 11 years to 21 years displayed behavioural change not attributable to an underlying medical condition3.
The frequency of these behavioural changes increased with age, with 50% of cats aged 15 or older reported to be affected. So why is this problem not more frequently reported to veterinarians? It is possible this discrepancy between prevalence and reported rates may simply be a consequence of owners either ignoring the behaviour or considering it to be a normal part of the ageing process and, therefore, not seeking veterinary attention. A Hill’s market research study reported, when specifically questioned, 75% of owners of dogs older than seven years of age reported their pet displayed one or more behavioural signs consistent with cognitive dysfunction, but only 12% of owners volunteered this information to their veterinarian.
As the focus on regular examinations of geriatric pets (to enable early detection of age-related illness) grows, veterinarians should consider specifically questioning owners to identify the development of signs of cognitive dysfunction.
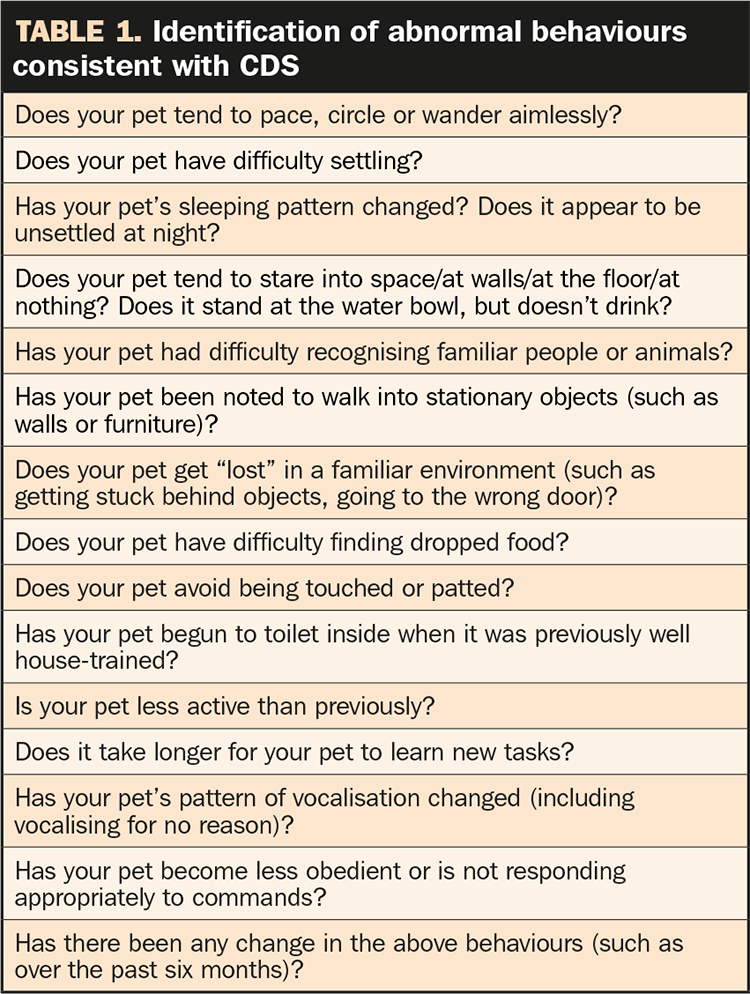
Examples are listed in Table 1, which shows questions to be asked of owners at routine elderly pet examinations that may assist in determining whether further investigation into underlying medical conditions and/or CDS should be undertaken.
As ageing-induced changes in the brain are considered to be irreversible4, it is preferable to identify any evidence of cognitive dysfunction early and take steps to slow or prevent the advancement of neuronal degeneration.
Ageing is associated with structural brain change in multiple species.
Pathological, immunohistochemical and advanced imaging investigations – comparing young and aged animals – have identified gross and microscopic changes consistent with neuronal loss or damage. Total brain atrophy with widening of sulci and ventriculomegaly has been identified in postmortem and MRI studies of normal and CDS aged dogs5,6,7. Specifically, it has been reported decreases in frontal lobe volume and hippocampal volume – areas of the brain associated with behaviour, learning and memory (Figures 1 and 2) – were found to precede decreases in total brain volume in beagles5. Significant changes in brain volume were evident from eight years of age in these dogs.
The exact cause of CDS remains unknown. However, based on the results of trial treatments and histopathological studies, it is suspected multiple factors may contribute to neuronal damage and loss. It is, therefore, these mechanisms that are the focus of treatments to counter CDS progression.
Oxidative damage to the brain may result from a combination of factors. Loss of protective antioxidant mechanisms8,9 and mitochondrial dysfunction10 have been reported to be evident in aged canine brains, and have been associated with increased production of reactive oxygen species. Subsequent oxidative damage on a cellular and nuclear level has been found to correlate with canine cognitive dysfunction11, with significant differences between affected and age-matched control dogs12.
Compromised blood (and, therefore, oxygen) flow to the aged brain may result from systemic illness (such as hypertension, cardiovascular disease and altered blood viscosity) or be a consequence of intraparenchymal vessel changes. Fibrosis of blood vessel walls was identified in 75% of dogs aged 8 years to 18 years, with cerebrovascular amyloidosis, microhaemorrhages and hyalinosis also identified6. The brain has the highest energy demand of all the body’s organs, and within the brain the neurons are most sensitive to metabolic imbalance13. Therefore, neurons are more likely to die as a result of ischaemic injury to the brain.
Neurogenesis is decreased in the hippocampus of aged (older than 10 years) dogs, with a reduction of 90% to 96% when compared to young adults (3 to 4 years old)4. This was hypothesised to be due to a combination of decreased progenitor cell proliferation, and the effects of reactive gliosis, stimulatory factors, hormones and neurotransmitters.
Beta-amyloid peptide accumulation is a hallmark of Alzheimer’s disease in humans, as well as normal canine and feline brain ageing14. These proteins may be neurotoxic, depending on the type of plaque formation15. The extent and location of these plaques have been associated with impaired cognitive function in dogs16–18.
Changes in the intracellular signalling pathways (such as the downregulation of brainderived neutrophic factor) can result in neuronal loss21.
Through specific cognitive testing protocols, mild impairment of visuospatial learning and memory functions have been detected in beagles by six years of age24, suggesting age-related brain degeneration may substantially precede the more overt behavioural changes witnessed by pet owners.
The incidence of CDS has been determined to increase exponentially with age older than 10 years, with 4.4% of 8-year-old to 10-year-old dogs – and 31% of dogs older than 14 years – affected in one large study25. Another study determined 68% of dogs aged 15 years to 16 years displayed impaired behaviour26. Interestingly, despite the fact dogs of smaller breeds tend to live longer than larger breeds, it has been found no evidence exists that age-related cognitive dysfunction progresses faster in different breeds1,26. No sex prevalence has been identified25,26.
Specific testing methods to evaluate various aspects of learning and memory in animals have been developed, but these are only available in laboratory settings. Therefore, in the general pet population, assessment of change in an animal’s cognition must rely on owner questioning to identify a deviation from what was previously normal.
A list of 27 behaviours that are significant in the differentiation of elderly dogs with cognitive dysfunction and those with normal ageing has been identified1. Further refinement of this data allowed development of a canine cognitive dysfunction rating scale (CCDR), whereby the severity of the change/abnormality seen was used to provide a total score25. A score of 50 or above was considered to differentiate between normal ageing behaviours and canine cognitive dysfunction, with an overall diagnostic accuracy of 99.8%. An online calculator of the CCDR is available at www.maturedogs.com
In cats aged between 11 years and 14 years, changes in social interaction have been reported to be the most prevalent clinical sign, while changes in the sleep-wake cycle in cats older than 15 years – in particular, vocalisation at night – was most commonly reported3. Aimless activity has also been reported to be frequently seen in this older cat population27.
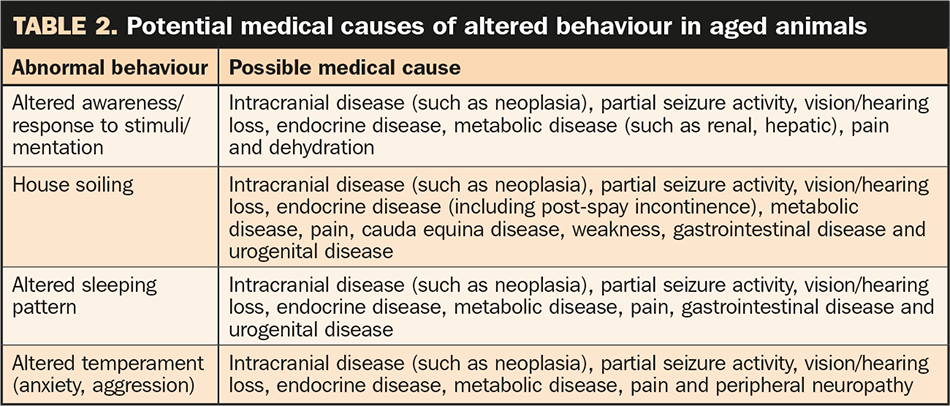
It is important, however, to consider behavioural change may be the result of an underlying medical condition, and patients should be evaluated appropriately to rule out other causes of altered behaviour. Potential medical causes of abnormal behaviour are listed in Table 2. Primary behavioural conditions and stress must also be considered.
Physical and neurological examinations (including assessments of vision, hearing and orthopaedic function), full haematology and biochemistry profiles, blood pressure measurement, urinalysis (including full routine analysis, urine protein:creatinine ratio and bacterial culture and sensitivity) and, potentially, imaging techniques and behavioural modification should be performed. Any medical conditions should be treated and thus ruled out as a contributing factor before a presumptive diagnosis of CDS is reached.
Due to its high rate of oxidative metabolism, high lipid content and limited ability for regeneration, the brain is considered to be particularly sensitive to the effects of reactive oxygen species. Therefore, these have been the target of multiple investigations into the effects of supplemental antioxidant compounds on histopathological brain features and cognitive function of aged mammals, including dogs28–31.
Subsequently, a commercial canine diet was developed, aimed at improving cognitive function in elderly canines through supplementation with antioxidants, L-carnitine and omega-3 fatty acids. When compared to dogs fed a nonsupplemented diet, improvement in cognitive function was seen as early as two to eight weeks, and maintained over two years32,33.
The exact mechanisms by which these supplements counter the effects of cognitive decline have not been fully identified, but potentially may be the result of a combination of factors. It has been suggested these treatments may decrease the production of beta-amyloid plaques34, improve lipid metabolism in the brain35, promote the survival of new neurons4, increase levels of brain-derived neurotrophic factor36 and maintain mitochondrial homeostasis10.
Long-term dietary supplementation with medium chain triglycerides (MCT) has also been found to improve cognitive function in aged dogs37. MCT are converted to ketone bodies by the liver and act as an alternative energy source to glucose, thereby countering the deficits in cerebral glucose metabolism.
Multiple non-pharmaceutical therapies are commercially available and aimed at improving cognitive function through the use of various combinations of dietary supplements, including antioxidants, vitamins, fatty acids, L-carnitine, co-enzyme Q10, and phospholipids (phosphatidylserine). A list of commonly used supplements is provided in Table 3, and their effects in Table 4. It is important to note compounds containing alpha lipoic acid should not be administered to cats, given the risk of toxicity in this species38.
Investigations in humans, rats and dogs have demonstrated stimulation of the brain (through social, environmental, physical [exercise] and cognitive enrichment) can slow the age-related loss of neurons in the brain.
In fact, behavioural enrichment alone has been shown to promote neurogenesis39,40. In our patients, this may be achieved through provision of toys, social interaction, exercise and training. Avoidance of stress should be paramount, and owners should be careful to tailor any physical activity to a level appropriate for their pet’s age, breed and any concurrent medical conditions.
Few medications are licensed for treating CDS, and data available on the use of human medications in veterinary patients is limited. The aims of treatment are to slow the rate of cognitive deterioration and ameliorate clinical signs.
Selegiline is a selective and irreversible monoamine oxidase B inhibitor in the dog41. It is thought to act by enhancing dopamine and other catecholamines in the cortex and hippocampus, as well as acting directly and indirectly as an antioxidant. A large clinical trial demonstrated improvement in specific cognitive testing, the sleep/wake cycle, activity level, the level of disorientation and social interaction of dogs with CDS by 30 days to 60 days following initiation of treatment at 0.5mg/kg to 1.0mg/kg once daily42. In this study of 641 client-owned dogs with CDS, 77% of the dogs were reported to show an overall improvement in clinical signs by day 60 on the basis of owner interpretation. Furthermore, selegiline has also been suggested to improve longevity of relatively healthy dogs aged 10 years to 15 years43.
While selegiline is not approved for use in cats, anecdotal off-label use has been reported to be effective at 0.25mg/kg to 1.0mg/kg every 24 hours27,44. Concomitant use with other monoamine oxidase inhibitors, tricyclic antidepressants, narcotics and selective serotonin reuptake inhibitors should be avoided, and care should be taken if the patient is receiving medications that may enhance serotonin transmission (such as tramadol). Clients should be advised it may take two weeks or more for beneficial effects to be seen.
Propentofylline is a xanthine derivative reported to improve cerebral perfusion by increasing cerebral blood flow, inhibiting platelet aggregation and thrombus formation, improving the flow properties of erythrocytes and providing bronchodilation45,46. It is licensed for treating dullness, lethargy and depressed demeanour in old dogs at a dose of 2.5mg/kg to 5mg/kg orally every 12 hours. It has also been reported to be used anecdotally and off-label in cats at 12.5mg orally every 24 hours27,44.
Nicergoline, an ergot derivative with alpha-one and alpha-two adrenergic agonist properties, is another medication that acts to increase cerebral blood flow, inhibit platelet aggregation, and provide neuroprotection and antioxidant effects47. The reported dose range in dogs is 0.25mg/kg to 0.5mg/kg every 24 hours (in the morning). Off-label use in cats has also been reported, at 1.25mg administered q24 hours44,48.
In some cases, it may be necessary to target treatment at countering specific clinical signs. It may be possible to promote sleep at night by increasing daytime activity levels through the administration of noradrenergic agonists, such as adrafinil and modafinil. Adrafinil administered at 20mg/kg every 24 hours has been reported to significantly increase locomotion in a trial population of aged dogs47, but reports on its effects on learning and memory are variable. Melatonin may be used to promote sleep and can be administered at 3mg to 9mg in dogs, and 1.5mg to 6mg in cats, 30 minutes prior to bedtime49.
Anxiolytics or medications that promote sedation may also be considered, with reports of the use of benzodiazepines, phenobarbital and gabapentin49. Care should be taken in the use of benzodiazepines in elderly patients due to their effects on the liver.
Oral diazepam should be strictly avoided in cats, given the risk of fulminant hepatic necrosis – however, clonazepam has not been reported to result in hepatotoxicity in this species. It is also important to note that tolerance to benzodiazepines can develop, resulting in a loss of efficacy.
The use of pheromones or other natural compounds may potentially assist in decreasing anxiety and enabling pets to settle at night.
Investigations into other pharmaceutical treatment strategies (such as hormone replacement therapy and medications to enhance cholinergic transmission) have not yet provided sufficient data on efficacy and safety to warrant their use on a clinical basis.
It is important to note the mechanisms of action of the above treatment options appear to be different in their treatment of age-related cognitive decline in people and animals, supporting the use of multimodal therapy over a single treatment protocol.
CDS may result in marked behavioural change, yet is considerably underdiagnosed in a veterinary setting. It is suspected owners perceive these abnormal behaviours to be a normal consequence of ageing, rather than a condition that may be improved with appropriate therapy.
Therefore, specific questioning of clients regarding such behavioural changes should be undertaken as part of a routine examination of elderly pets. Potential underlying medical conditions should always be ruled out prior to initiating treatment for CDS, but, once undertaken, multimodal therapy (including dietary modification/ nutraceutical administration, environmental enrichment and potentially pharmaceutical intervention) should be pursued to provide the greatest health benefit.