14 Sept 2022
Silvia Cugat PgCertWBIS, MRCVS, Oliver Marsh BVM, BVS, MRCVS and Ane Uriarte DipECVN, DVM, EBVS, MRCVS share a case report outlining a successful surgical procedure in a 17-year-old cat

Image: © jagodka / Adobe Stock
The objective of this case report is to describe the successful surgical treatment and outcome in a senior cat that had undergone cranioplasty with titanium mesh to repair bilateral skull fractures.
Two malleable titanium meshes were shaped to cover the bone defects of the parietal and occipital bones, and were fixed with cruciate and self-tapping titanium mesh screws. The skull structure and cosmetic appearance of the patient were successfully restored, providing the patient with robust brain protection and a return to normal activity.
Although this surgical treatment had a positive outcome in this patient without postoperative complications, we encourage further research with larger study samples before drawing this conclusion for the general population of cats with calvarial fractures.
A 17-year-old outdoor female neutered domestic shorthair cat was referred to the neurology department at Southfields Veterinary Specialists with a seven-day history of abnormal mentation and blindness. Previous to the mentioned neurological signs, she had been found by the carers with possible signs of having been in a fight.
On presentation, she was circling to the left without proprioceptive deficits and had absent menace response in the right eye with normal pupillary reflexes. The remaining physical examination was unremarkable and a skin lesion on the head was not found.
She was admitted for investigation into left forebrain lesion. Given the differential diagnoses of brain tumour and inflammatory granuloma (bacterial or protozoal being the most likely), permission was given to perform blood work, thoracic radiographs and MRI of the head under general anaesthetic.
The cat was premedicated with methadone (0.1mg/kg) and maropitant (1mg/kg), and induced with propofol (2mg/kg to 4mg/kg IV, to effect). Anaesthesia was maintained with isoflurane and oxygen. Standard fast‑spin echo sequences were used in a 1.5‑Tesla MRI scanner (Figure 1).
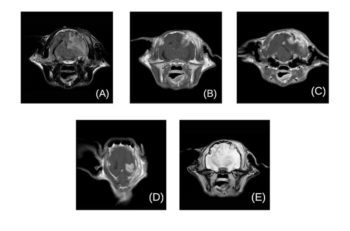
The brain sequences revealed two lesions: a large 10mm (H) × 8.6mm (W) × 11mm (L), rounded, well defined, intra-axial mass lesion located in the left parietal lobe and in contact with the overlying skull. Focal tissue swelling of the temporal musculature was also identified.
To a lesser degree, similar skull and soft tissue changes were also visible at the same level on the right side of the calvarium, with the presence of a second, smaller mass of similar characteristics (4.6mm × 3.7mm × 2.8mm).
The left-sided mass was producing a moderate mass effect, with marked rightward midline shift, and severe T2‑weighted and fluid‑attenuated inversion recovery hyperintensity of the white matter surrounding the lesion, extending from the frontal to the occipital lobe, consistent with vasogenic oedema.
Additionally, mild dilation of the ventricular system was observed with distension of the olfactory recesses, regional meningeal enhancement compatible with meningitis and caudal transtentorial brain herniation.
Based on the MRI findings, despite not detecting a healed wound during physical examination or a primary bacterial focus of infection on radiographs, a penetrating trauma (such as a fox bite) was thought to be the cause of the skull fractures and secondary bilateral abscess.
The cat was taken to surgery under the same anaesthesia. Following an increase in blood pressure and consequent bradycardia, also known as the Cushing or vasopressor response, cordycepic acid 1g/kg and dexamethasone 0.15mg/kg were administered to reduce perilesional oedema and intracranial pressure.
A fentanyl bolus and constant rate infusion (CRI) were administered intraoperatively at 1mcg/kg and 0.2mcg/kg/min respectively, which ended during recovery. In addition, a medetomidine bolus (3mcg/kg) and CRI was started (1mcg/kg/h), and then reduced postoperatively (0.5mcg/kg/h). IV fluids were given at 3ml/kg/h throughout surgery. The cat was placed in sternal recumbency and the head was kept elevated above the level of the heart, using a padded sandbag, to promote venous outflow.
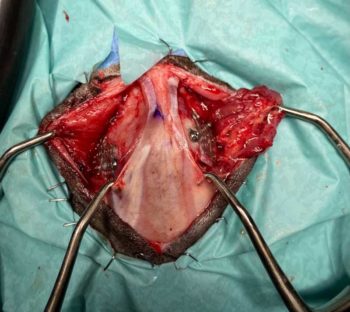
A bilateral temporoparietal craniectomy was performed through a rostrotentorial approach (Figure 2). The skin and subcutis were incised in a bilateral U-shape starting over the frontal bone and passing to the parietal towards the occipital bone. The underlying soft tissue was inflamed and abnormal.
Each temporalis muscle was incised close to its attachment to the skull, and elevated and retracted laterally to expose the bilateral fractures. Care was taken to avoid damage of the auriculotemporal nerve and the vascular supply bilaterally. Each fracture was enlarged using a high-speed pneumatic drill with a 4mm burr to allow access to the affected brain. Purulent material was observed when the area was explored.
Following bacterial swab collection, the penetrating bone fragments were removed and the cortex was decompressed. The surgical sites were flushed and debrided, particularly the surrounding soft tissue that was also infected. Mesh cutters were used to cut two custom-made malleable titanium meshes (40mm × 50mm and 0.6mm thickness) slightly larger than the craniectomy sites and then shaped manually according to the contours of the cat’s skull. A 1mm diameter drill bit was used to drill two guide holes on each side of the surrounding temporoparietal periosteum. The aim was to reduce the chances of cracks forming when used close to the fracture lines.
A cruciate screwdriver was used to secure two low-profile, 3mm tapered cruciate titanium screws to the mesh until the closure of the defects was achieved. Routine closure was performed by layers.
IV antibiotics (cefuroxime 20mg/kg and metronidazole 10mg/kg) were given preoperatively and perioperatively, then continued orally postoperatively every 12 hours for a total of 10 weeks. The cat was neurologically normal after recovery and remained so during the follow-up periods at 5 and 12 months postoperatively. No post-surgical complications were observed during this time.
Brain abscess has rarely been reported in dogs and cats, with an incidence below 3%1. The most common suggested aetiologies are penetrating injuries (such as a foreign body or bite wound), as a complication of immunocompromised animals1-6, and spread from local or distant primary infections (such as otitis, bacterial endocarditis or lung granuloma).
Patients typically present with signs secondary to mass effects3,7, such as identical neurological signs to those of brain neoplasia (for example, changes in behaviour, mental status, visual and postural reaction deficits), which may lead to a presumptive diagnosis of brain tumour8.
Since early detection and treatment of brain abscess can result in better outcomes, before determining whether euthanasia should be performed, an MRI is recommended to aid differentiation between neoplastic and non-neoplastic brain diseases9.
Management of a brain abscess is considered a surgical emergency – especially when a mass effect displacing normal brain tissue and causing cerebral hypoxia is present.
A multidisciplinary approach is preferred in cases of traumatic brain abscessation where intracranial hypertension is present; analgesia, IV antimicrobials and removal of infected bone fragments, followed by copious lavage of the area are advised3,10-12. The surgical goal is to decompress the brain and prevent herniation, which results in improved brain perfusion and compliance.
In humans, surgical treatment may vary depending on the abscess size, location and stage of cerebritis.
The gold standard is the use of minimally invasive surgery through stereotactic aspiration and drainage, which allows for reduction of the mass, subsequently reducing intracranial pressure, as well as offering another diagnostic tool8.
Surgical excision is still necessary, however, if the abscess diameter exceeds 30mm, if the abscess stage is too advanced or if residual recurrence remains13,14.
In small animals, a successful case report using stereotactic drainage to treat an inoperable brain abscess in a cat has been documented5. However, since this surgical technique has been associated with abscess recurrence in humans15, the potential for increasing anaesthetic risks and costs when repeating this procedure should be carefully considered.
In the present case, neurological deterioration, intracranial hypertension, and the presence of penetrating infected fractures and purulent material within the brain parenchyma were indicators that our patient required a craniectomy.
Nevertheless, concerns were raised about the significant bone loss that will remain following a bilateral craniectomy.
After surgical decompression, the main goals were to restore skull function, protect the brain and provide structural support in an aesthetically acceptable fashion.
Several techniques and materials are available for skull reconstruction following a destruction of the bone secondary to a trauma, infection or tumour infiltration. The most common technique used in dogs and cats with brain abscess is the temporal flap, which involves leaving the temporal or parietal bones open, allowing drainage, then covering the defect with healthy temporal muscle fascia1-3,11,12. However, this technique could have exposed the brain to further trauma in the authors’ cat, which presented two large bone defects.
In the reviewed literature, a variety of surgical materials – such as bone allografts16,17, porcine intestinal mucosa flap with polypropylene mesh4, and titanium mesh17‑19 – were used in small animals requiring cranioplasty.
These materials differ in osteogenesis, osseointegration, osteoconduction and osteoinduction properties, impacting healing and regeneration of the bone defect. Moreover, they vary in their functionality, aesthetic effect, cost and surgical complications associated with their use.
Retrospective studies in domestic animals comparing cranioplasty materials and the risk associated to their use could not be found. Nevertheless, a meta-analysis review of 1,003 cases comparing the complication rates of the most common prostheses used for cranioplasty in human surgery reflected that infection was the most prevalent complication rate; 17.44% for autologous bone, 14.1% for polymethylmethacrylate (PMMA), 8.6% for titanium and 5.88% for ceramic. Finally, the combination of titanium with PMMA led to 0% infection rate20.
In this report, the number and size of the skull defects, the extended surgical time to make two different moulds and the risk of causing bone necrosis influenced the decision not to use PMMA. When the bone availability is limited, titanium mesh is the preferred biocompatible replacement to repair calvarial fractures and defects after tumour resection in dogs17,18,21,22.
Due to lack of literature in felids, it was the authors’ decision to extrapolate this resolution towards the cat described. The decision‑making process was also based on the features of the titanium mesh applied to this clinical scenario. It was thought that a lightweight and flexible implant would facilitate contouring intraoperatively, and that the robust composition and self‑tapping titanium screws would ensure great stability17,18.
Additionally, the integration of the mesh with the temporal muscle would prevent its collapse and adhesion on to the brain, providing optimal functional and cosmetic results.
The authors’ case report had some limitations. Only one case has been described retrospectively; therefore, significant statistical relevance could not be provided as a result of the small sample size.
Although imaging reports and macroscopic examination of the purulent material within the brain was highly suggestive of brain abscessation, histopathological analysis would have been of greater diagnostic value since drawing cultures post-antibiotic administration may decrease the blood culture yield1,3.
While MRI is not available in all veterinary practices, CT is becoming more accessible and, although less sensitive than MRI, it can be used as an effective diagnostic tool for brain abscesses1,2,23.
Finally, costs might be considered another limitation for its use; however, its multiple features, as previously discussed, outweighed this disadvantage.
In summary, additional research is required to further evaluate the benefit of using non-absorbable implants after large skull defects in cats. Nevertheless, we believe the implant choice was appropriate for this patient in that it allowed the provision of excellent protection to the brain, while allowing functional and aesthetically satisfactory restoration without infection-associated complications.
To the authors’ knowledge, this is the first report of successful surgical treatment of a presumptive traumatic bilateral intracranial brain abscess in a cat by the use of titanium mesh.
According to this case report, the rapid resolution of neurological signs and short hospitalisation period without postoperative complications were indicators that titanium mesh was a successful treatment for repairing the skull bone defects in this cat.