9 Apr 2018
Karen Walsh considers methods of assessing pain, drugs used to relieve the condition and ways of administering them.

The International Association for the Study of Pain has defined pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage”. Pain is, therefore, a subjective emotion.
The difficulty in veterinary medicine (and non-verbal humans) is the inability of these patients to verbalise such emotions and, in 2001, a note was added to the definition of pain. This states: “the inability to communicate in no way negates the possibility an individual is experiencing pain and is in need of appropriate pain-relieving treatment”.
As veterinary carers, it is our duty to be vigilant to the signs of pain and discomfort, and be the advocates for animals under our care.
Several types (nociceptive, inflammatory, neuropathic and functional, cancer and post-surgical pain) and classifications (acute versus chronic pain, somatic versus visceral, superficial versus deep) of pain exist that reflect the complexity of this condition.
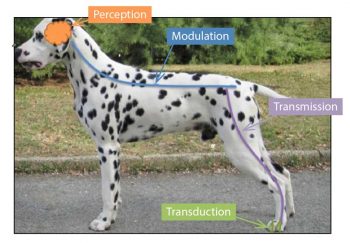
The pathways involved in producing pain occur in four main steps. A noxious/painful event (mechanical, thermal, electrical or chemical) stimulates peripheral receptors, and these are transformed (transduction) into electrical signals, which are transmitted (transmission) to the spinal cord where they are modulated (modulation) then projected (projection) to the brain, where they are finally processed and produce awareness of that painful stimulation (perception).
As pain is an emotional experience it is impossible to have a purely objective measurement of it. Heart rate, respiratory rate and blood pressure may add to the picture of a patient in pain, but these measurements can also be influenced by stress and illness. Cats find the visit to a veterinary practice stressful, so they can present even greater challenges when assessing pain and comfort.
When assessing pain in animals it is very important to include physiological and behavioural changes. It is important to assume animals are closely related to humans in pain perception; therefore, remember, if it hurts you it may hurt them.
When assessing pain, several behavioural aspects need to be considered, which include posture, mobility, activity, response to touch, attention to the painful area and vocalisation.
Every animal will demonstrate pain in a unique way and knowing the behaviour of that animal before the painful stimulation can be of significant help in identifying pain. In fact, behavioural and physiological responses to pain are widely affected by illness, species, breed, age, temperament, the severity and duration of pain, and drug administration.
Sometimes pain is difficult to identify, so the response to an appropriate pain killer remains the best marker for accurate diagnosis of pain.
Several categorical ways of assessing pain have been suggested – many focusing on dogs, but some tools have been developed for cats. It is important to understand all these scales are, to some extent, subjective, prone to error and subject to some degree of variability between assessors.
Pain scales should be used as an adjunct to physical evaluation and it is important to ensure pain is assessed and treated in every patient. In cases where the clinician is still unsure a test dose of analgesic should be administered, and the patient’s response should be assessed.
If the patient is sedated or critically ill, it may not be able to express adequate pain behaviours and, again, a test dose of analgesics should be administered. Increased awareness to surroundings can be considered as a positive response to analgesic treatment.
Composite and multidimen-sional pain scales were initially developed to assess acute pain in dogs (and in laboratory rats), but studies have begun developing tools for use in cats. The composite and multidimensional scales have in common the fact they evaluate and score several categories (behavioural, physiological and contextual).
This type of scale is, therefore, more complex, which makes them time-consuming, but as the observer becomes more familiar, time taken to make the assessment will decrease. The observer only confirms the presence or absence of the behaviour – increasing accuracy and reducing bias.
This scoring system is the first composite scale to include facial expression evaluation. Facial expression has been used to assess pain and response to analgesia in non-verbal humans for some time, and long-standing interest has existed in using this method to assess pain in feline patients.
Both these scoring systems can be downloaded via https://bit.ly/2GfjESo
The Colorado State University produced a pain scale that can be used to assess acute pain in dogs and cats. This scale considers the psychological and behavioural elements, response to wound palpation and body tension. The unique characteristic of this scale is it uses generic 0 to 4 scoring along with a colour scale. Also, additional drawings provide space for recording pain, warmth and muscle tension – allowing a more specific documentation.
Unlike all other scales, it considers the residual effects of anaesthetics (non-assessment in the resting patient). Unfortunately, this scale is not validated and it does not have a set intervention level.
This pain scale uses behavioural as well as physiological information to assess pain level in cats undergoing ovariohysterectomy. It was originally published in Portuguese, but later translated to English. It does require blood pressure assessment before and after the surgical intervention, which may mean it is less applicable to general practice.
None of these scales are perfect; however, they provide the practitioner with a tool to compare levels of pain over time or discuss the patient between colleagues.
In general, it is helpful to have some degree of pre-planning regarding the invasiveness of an elective procedure. This will allow the pre-emptive or preventive use of analgesics, which will help decrease the overall level of pain.
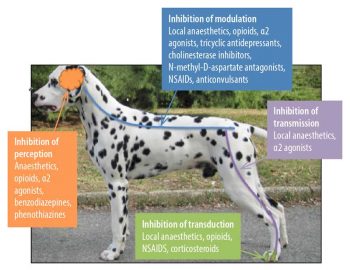
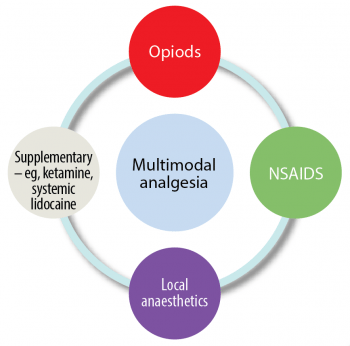
Many studies have shown if we use pain killers from more than one group of analgesics that target the pain pathways (Figure 1) at different levels, the effect will be more profound and lower total doses of each individual drug will be used. The advantage is the side-effects may be reduced. This is called multimodal analgesia (Figure 2).
Preventive analgesia is a method of averting or reducing the central sensitisation that results from a painful insult and the inflammatory reaction that develops after it. The term “pre-emptive analgesia“ describes mainly the timing of pain intervention (before versus after the insult). Preventive analgesia covers the entire period over which central sensitisation may occur (Clark, 2014). Its use was controversial and proving its relevance in clinical trials was difficult.
Multimodal analgesia in the perioperative period is the cornerstone of successful prevention of central sensitisation, which, in turn reduces postoperative and chronic pain. This should be carried into the postoperative period.
Multimodal analgesia (Figure 3) uses drugs from separate groups of analgesics with the aim of improving the level of analgesia, potentially reduce overall doses of each drug and decrease side effects (Murrell, 2014).
We will often practise multimodal analgesia unwittingly when you administer an opioid and a NSAID when performing surgery. It is useful to think about the separate groups when deciding on an analgesic protocol.
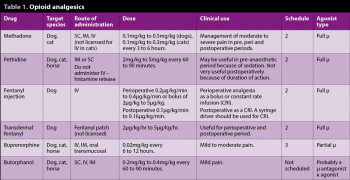
Opioids (Table 1) are commonly used in the perioperative period and often part of the premedication. They are useful in treating sharp pain and help decrease the brain’s perception of pain. Opioid receptors are also found in inflamed tissues, such as joints, which enables them to be used as local treatment. Some opioid side effects are:
As with any prescribed drug, it is important to consider those licensed for use in that species. In the UK, licensed preparations of methadone and buprenorphine exist in the dog and cat. Fentanyl (IV) is licensed in the dog only.
The author prefers to use a full agonist in most surgical procedures because it gives more flexibility when administering further drugs during surgery. The patient can receive methadone or buprenorphine in the postoperative period.
Short-acting drugs, such as fentanyl, are best suited to continuous analgesic therapy. These drugs have a rapid onset of action and a short duration of action that allows the effect to be switched on and off when needed. It tends to smooth out the plane of anaesthesia and reduce the amount of inhalational agent required (Ueyama et al, 2009). A syringe driver should be used to allow accurate administration. As fentanyl is licensed for IV use in dogs, it should be used in preference to other short-acting opioids.
The facility to perform intermittent positive-pressure ventilation should be available as respiration may be compromised. The patient should also be monitored for bradycardia and the dose titrated to effect. The inhalational agent will often be able to be decreased and this will help to maintain blood pressure within acceptable limits.
Longer-acting drugs, such as methadone and morphine, can also be administered as constant rate infusion (CRI), but, as they are longer acting than fentanyl, they are less easy to titrate to effect and are possibly more suited to postoperative analgesia. If administered during anaesthesia they will decrease the inhalational anaesthetic requirements.
Local administration of opioids allows lower doses to be used with potentially fewer side effects.
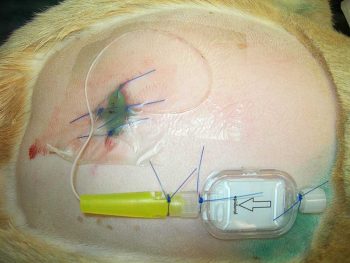
Morphine (0.1mg/kg) can be administered in saline or in combination with local anaesthetics at the lumbosacral junction to produce analgesia up to the thorax. This can be administered as a single injection or continuing into the postoperative period (morphine plus saline only) if an epidural catheter is placed (Figure 4). It should be diluted with sterile saline to increase the volume and aid distribution or it can be mixed with local anaesthetic. The advantages are:
The disadvantages are:
Spinal or intrathecal administration can be achieved by injection or as a splash application during surgery. The dose is usually reduced compared to epidural administration (0.05mg/kg compared to 0.1mg/kg morphine).
Patients with an ongoing inflammation in a joint will have an increase in opioid receptors. This can be exploited to achieve pain relief in the affected joint without systemic administration of the opioid. A dose of 0.1mg/kg morphine is often recommended. Although improvements in pain scores have been recorded it did not significantly improve force plate pressures in one study (Gurney et al, 2012).
For epidural, spinal and intra-articular administration, preservative-free morphine should be used as the preservatives may affect the tissues adversely.
NSAIDs are one of the most important groups of pain-relieving drugs in acute and chronic pain as they will target the specific reason for pain – inflammation, either primary or secondary. It is important to use the appropriate drug and route of administration for the species.
Fewer NSAIDs are available for cats compared to dogs. As with all drugs they have side effects and it is important to look out for signs that might signify a problem. Cats are less likely to show overt vomiting when gastrointestinal (GI) side-effects occur – inappetence being the most likely sign.
Strategies that can be used to improve safety when using NSAIDs are:
In patients that the practitioner can measure blood pressure and treat any hypotension, administration of NSAIDs before anaesthesia is the ideal. If the clinician suspects blood pressure may possibly be compromised during anaesthesia – for example, septic patients and procedures associated with severe blood loss – administration post-anaesthesia may be the least problematic.
Local anaesthetics may be used to block specific nerves to stop transduction of the painful stimulus onward to the spinal cord. When planning a surgery, think about the possible local anaesthetic blocks available or the application of anaesthetic drugs to the wound site.
Using this group of drugs will help to reduce the requirement for morphine-type drugs. Calculate the total amount of drug that can be used and divide it between different sites. As the volume can be small it may need to be diluted with saline to make a usable volume.
Local anaesthetics can be combined with opioids (as mentioned previously) or other drugs, such as adrenaline and α2 agonists. The latter two have been shown to increase duration of action of local anaesthetics.
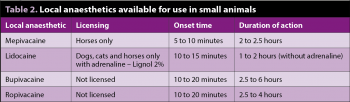
As with all anaesthetic drugs, toxicity and complications can occur when using local anaesthetics. Careful calculation of volumes to be used, particularly in smaller patients, are important to minimise the likelihood of high plasma levels (Table 2).
It has become common for people to mix local anaesthetics of varying durations and onset of action – for example, bupivacaine and lidocaine. When two agents are mixed this may result in an alteration of pKa, as well as altering the concentration of drugs used making prediction of onset time difficult.
Evidence shows this practice has little clinical advantage, as often the duration of action of the longer-acting drug is shortened (Gadsden et al, 2011).
Lidocaine has been administered as a CRI to provide analgesia perioperatively and postoperatively. Systemic administration of lidocaine has become increasingly popular over the past decade. It appears to be more effective against visceral pain, so may have a place in ophthalmic pain treatment.
A pilot study by Smith et al (2004) found a loading dose of lidocaine (1mg/kg) followed by a CRI (25mcg/kg/min) was equipotent to morphine (0.15mg/kg followed by 0.1mg/kg/hr).
Other studies have also shown lidocaine CRI decreased inhalational requirements in a dose-dependent fashion without significant changes in heart rate and blood pressure. This technique may be of interest in practices that do not have easy access to controlled drugs. The disadvantages include the need to maintain IV access to administer this type of analgesia and the requirement for some type of syringe driver or fluid pump.
Ketamine is a dissociative anaesthetic agent that has profound analgesic activity at sub-anaesthetic doses. It is particularly useful for neuropathic (nerve) and what is called acute on chronic pain. Acute on chronic pain is when a patient experiencing chronic pain requires surgery on, or receives an injury to, that area.
Ketamine is a very important analgesic and anaesthetic drug in veterinary medicine, but its supply is under threat because of addiction problems in humans. In general, doses as low as 0.5mg/kg can be administered as a one-off bolus, which can be followed up with a CRI during surgery and the postoperative period: bolus dose 0.5mg/kg to 1mg/kg, CRI 2mcg/kg/min to 20mcg/kg/min.
These drugs are more commonly used as sedatives in cats and dogs, but also have very potent analgesic activity (Valtolina et al, 2009).
Due to the sedative and cardiovascular effects of these drugs, they are seldom indicated for use as a primary analgesic; however, they are useful in sedative combinations and can be used in patients experiencing pain that cannot be controlled with other analgesics. Analgesia appears to be dose-dependent and sometimes can be difficult to distinguish from sedation.
Pain assessment is of paramount importance for the welfare and comfort of dogs and cats. Several ways of assessing pain have been proposed and each one comes with its advantages and disadvantages. Whichever way is chosen to assess pain, it is important the observer is familiar with the pet’s behaviour.
Each clinic should develop protocols for the assessment of acute and chronic pain. It is also fundamental to keep in mind experience of pain can alter rapidly, and pain assessments should be performed frequently. Sometimes the best tool for accurate diagnosis of pain is the actual administration of an analgesic. The patient will need to be reassessed, analgesic efficacy will need to be re-evaluated and, finally, analgesia will need to be readministered appropriately as assessment requires.