27 Feb 2017
Postoperative pain management in companion animals: an update
Alberto Palella Gómez looks at assessing pain in small animals after surgery and latest approaches to its management with or without drugs.

Adequate postoperative pain control is very important to reduce complications and hospital stay. This also relies on a correct pain assessment of the patient. Pain score tools, such as behavioural pain scales, help us identify the appropriate time and level of intervention. Pre-emptive and multimodal analgesia have a key role in the correct management of pain. Multimodal analgesia comprehends pharmacological drugs, such as opioids, NSAIDs, local anaesthetics, analgesic adjuncts and non-pharmacological methods, such as cold compress therapy, manual therapy and good nursing.
Pain is a complex, multidimensional experience involving sensory and affective (emotional) components, which can be classified as acute or chronic.
To be adequate, postoperative pain management should encompass pre-emptive and multimodal analgesia.
Pre-emptive analgesia is the practice of preventive pain management by the principle of preventing upregulation of pain pathways and the development of central and peripheral sensitisation (Katz et al, 2011). This involves the use of analgesic drugs before the noxious stimulus occurs (or as soon as possible if it has already occurred) and continued use of these drugs afterwards for an appropriate period.
Data from studies on acute postoperative pain indicate it is greater in the first 24 hours following surgery, although persistent pain is present in a considerable percentage of individuals – even after common operations. For instance, in dogs and cats undergoing ovariohysterectomy, behavioural changes were observed for at least three days, suggesting pain relief is required following this procedure (Fox et al, 2000; Brondani et al, 2009).
Multimodal analgesia consists of administrating a combination of drugs belonging to different classes. These mainly include opioids, NSAIDs, local anaesthetics, alpha-2 agonists and non-pharmacologic analgesic techniques. Most drugs have a synergistic action and their combination will allow the use of lower doses to minimise side effects. The latest data suggests multimodal analgesia strategies – such as the combination of an opioid and NSAID – are already widely used for routine surgeries in the UK (Hunt et al, 2015).
Pain assessment and recognition
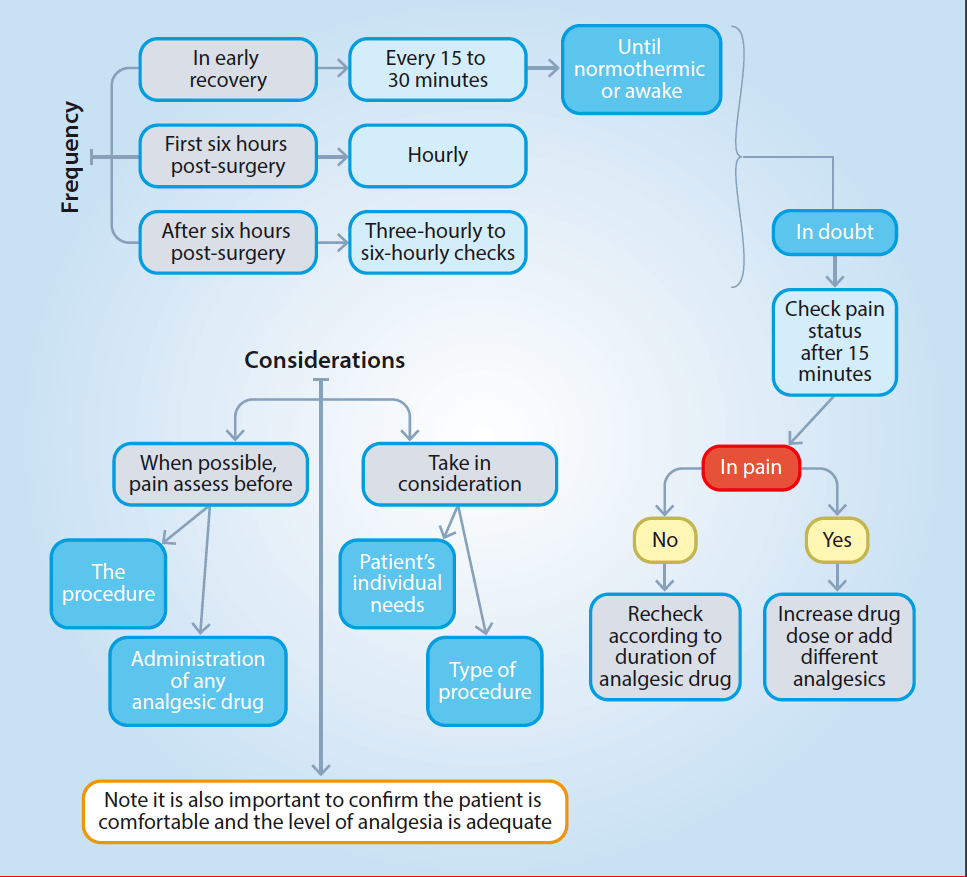
Effective management of pain relies on the ability to recognise it. For this purpose, pain assessments should be performed at intervals to judge whether the management is adequate and to avoid the underuse or overuse of analgesic drugs. The frequency of pain assessments and considerations are represented in Figure 1.
Objective measurements, including heart rate, arterial blood pressure, plasma cortisol, have been associated with pain in dogs (Hansen et al, 1997). However, these are unreliable as stress, fear, disease and anaesthetic drugs may affect these parameters in the same way as pain (Srithunyarat et al, 2016).
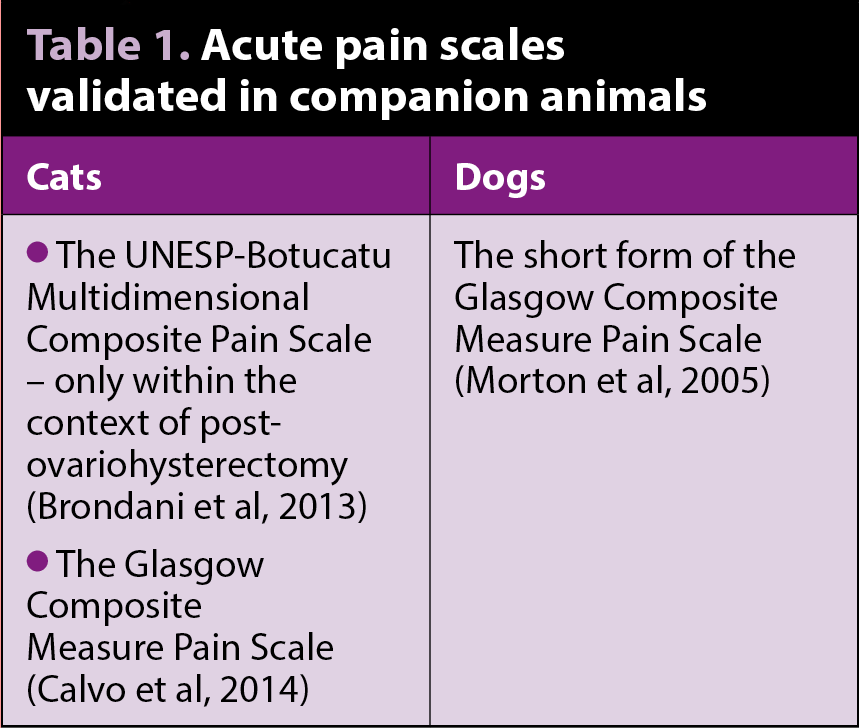
Scoring tools, such as pain scales based on behavioural changes, are very useful to record changes in pain over time or after intervention. Few pain scales are validated to assess acute pain in companion animals (Table 1).
Pharmacological management
Different analgesic drugs are available to manage postoperative pain. It is important to be aware of local licensing regulations and, if in the UK, to follow the VMD’s prescribing cascade.
Opioids
Opioids are already widely used in clinical practice in the UK, hence they will not be described in depth in this article. Opioids are the cornerstone of effective postoperative pain treatment as they have high efficacy and are remarkably safe (Pascoe, 2000). They vary in their receptor specificity, potency and efficacy, resulting in different clinical effects.
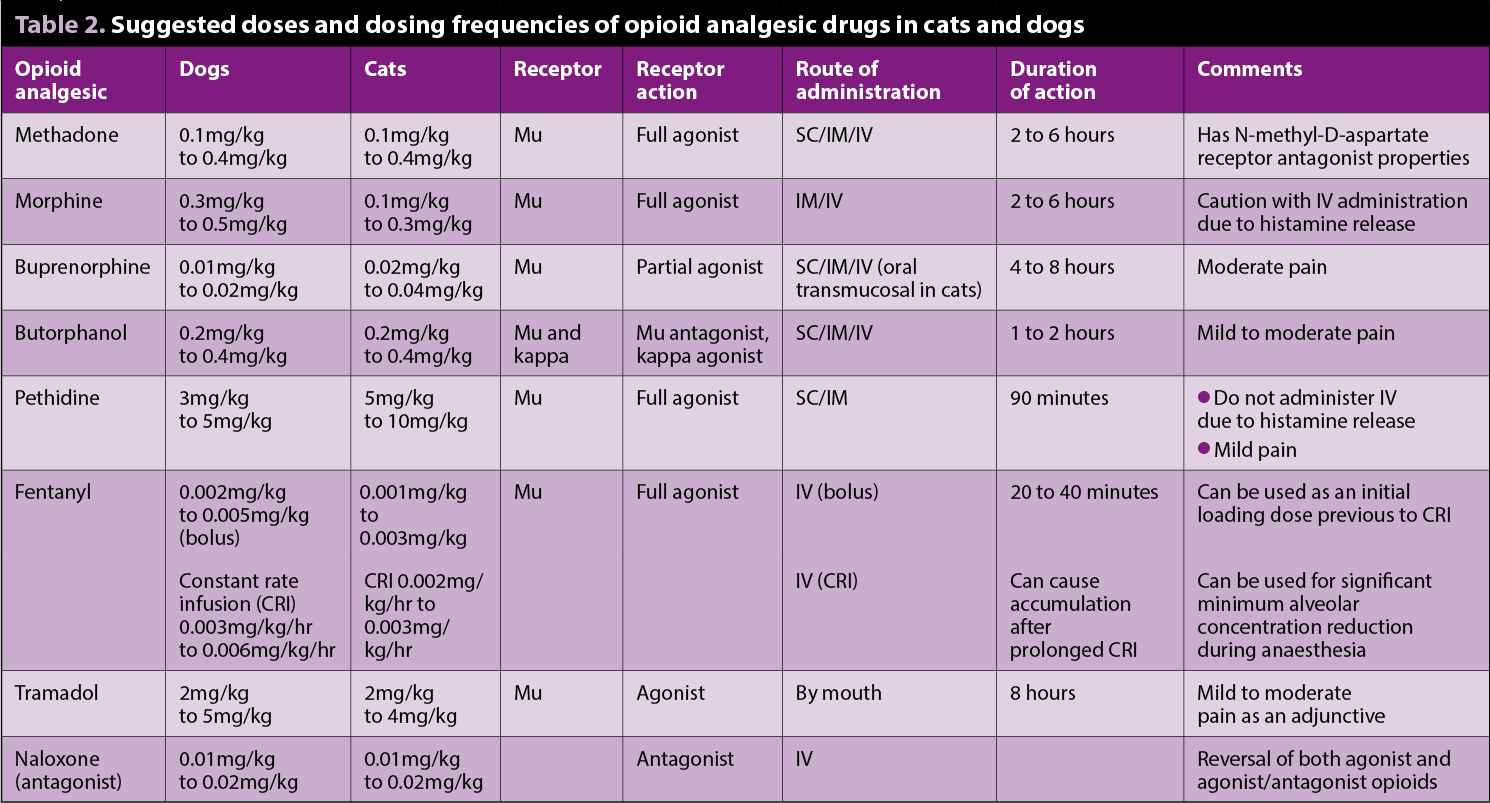
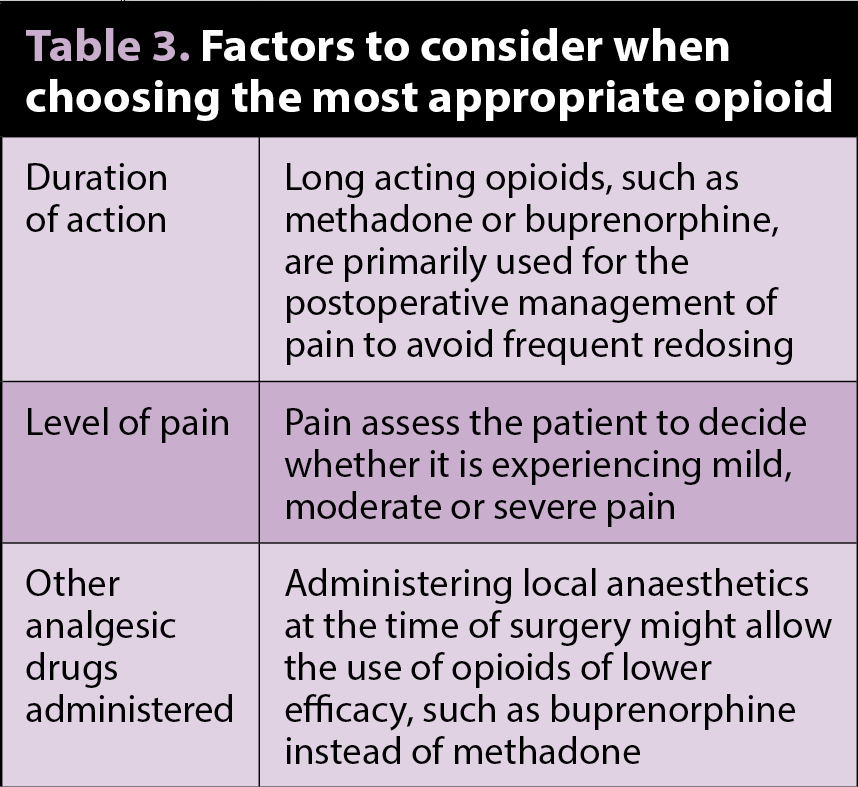
Several opioids are licensed for veterinary use, but it is necessary to be aware of indications and species variations (Table 2). Full µ agonists elicit greater and more predictable analgesia than partial µ agonists or k agonists. When choosing which opioid is the most appropriate to use, the factors shown in Table 3 should be considered.
The most common side effects of opioids are usually associated with excessive doses. These include vomiting (mostly with morphine administered in the premedication period), nausea, dysphoria, panting (atropine-responsive), bradycardia and respiratory depression. Any of these adverse effects can be prevented with careful titration of the drug and, if necessary, can be reversed with naloxone administered to effect. It must be kept in mind naloxone will also antagonise the analgesic effects of the first opioid.
In cats, buprenorphine administered via the oral transmucosal (OTM) route has been proven to be as effective as the IV route (Robertson et al, 2003; Robertson et al, 2005), but later clinical studies have given rise to some controversy on this matter and, where possible, the IV and IM administration routes are preferred to SC and OTM (Steagall et al, 2006; Giordano et al, 2010).
Fentanyl is a potent, synthetic μ agonist opioid with a rapid onset and short duration of action when administered IV. It is a Schedule 2 narcotic – approximately 100 times more potent than morphine. Its use as boluses or constant rate infusion (CRI) should be considered for its potent analgesia and minimum alveolar concentration-sparing effects.
A transdermal fentanyl solution was licensed for use in dogs, but has been removed from the market (although, at the time of writing this article, it may still be found). For this reason, the off-licence use of fentanyl patches – specifically designed for relief of chronic pain in humans – is becoming popular again.
A review article on transdermal fentanyl patches in small animals showed effective plasma concentrations were achieved in dogs approximately 24 hours after patch application and produced analgesia for up to 72 hours. In cats, effective plasma concentrations were achieved 7 hours after patch application and produced analgesia equivalent to intermittent butorphanol administration for up to 72 hours (Hofmeister and Egger, 2004).
NSAIDs
NSAIDs exert antipyretic, anti-inflammatory and analgesic effects. Unless contraindicated, NSAIDs are the mainstay of most analgesic protocols – being administered on their own in mild pain states or as part of a multimodal approach in more severe cases.
No data exists showing one NSAID is superior to another, and if one fails to provide analgesia, another type of NSAID can be considered. Caution should be taken when switching between NSAIDs and should follow a washout period of several days (Goodman et al, 2009).
Many NSAIDS are licensed for veterinary use with variations in species and condition indications. It is particularly important to check the product datasheets as some preparations may not be licensed in the perioperative period, where a reduction in systemic blood pressure related to anaesthesia and combined with constitutive cyclo-oxygenase (COX) inhibition (mostly those related with the kidneys and the intestinal tract) may increase the risk of adverse events (Monteiro-Steagall et al, 2013).
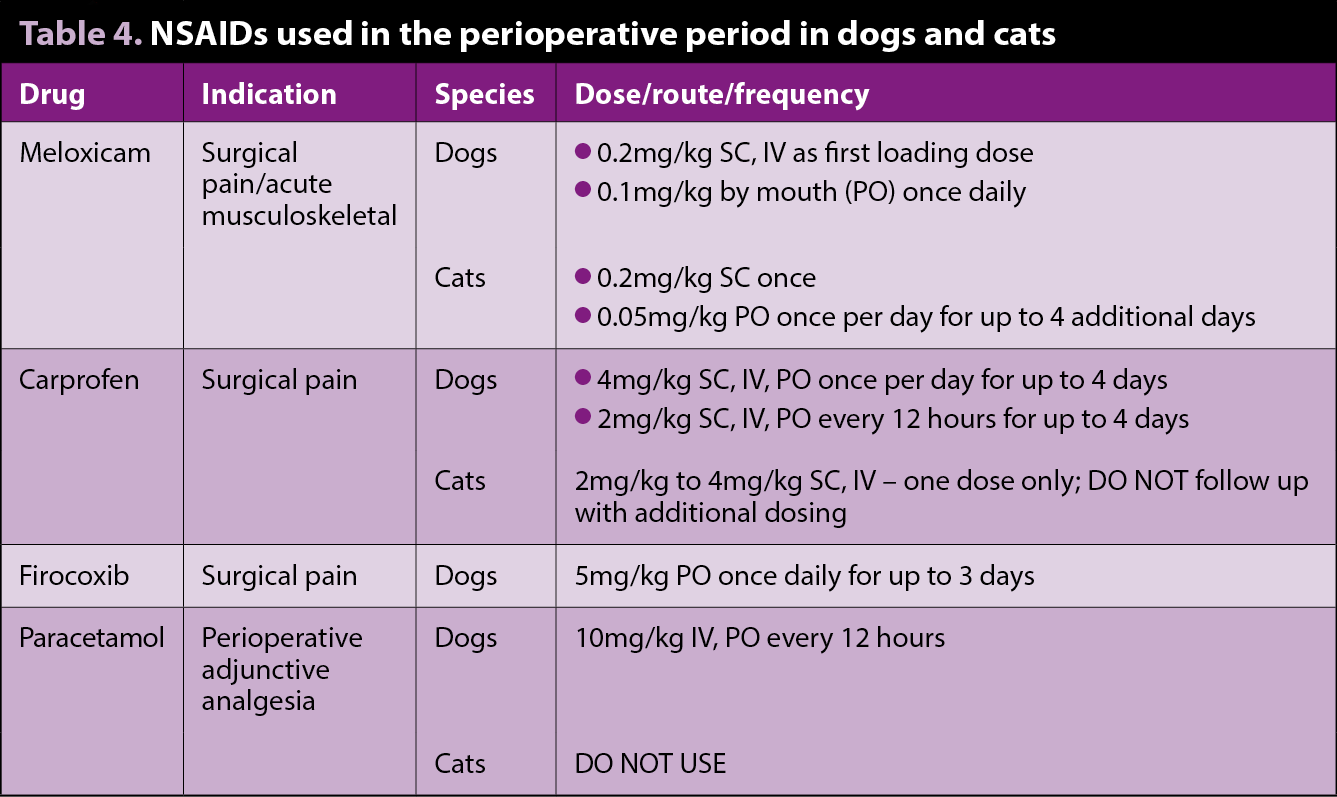
Although the use of NSAIDs is contraindicated in patients with kidney disease, a retrospective study on the effects of meloxicam on the longevity of old-aged cats with degenerative joint disease, and with or without chronic kidney disease (CKD), suggested meloxicam should be considered as a part of the therapeutic regime in cats with chronic painful conditions (titrated to the lowest effective dose – 0.02mg/kg in this particular study). Meloxicam did not appear to reduce the lifespan in patients with pre-existing stable CKD, nor for cats in International Renal Interest Society stages two and three (Gowan et al, 2012).
During the perioperative period the recommendation must be the patient is not dehydrated or hypovolaemic and that blood pressure is maintained. When administered before anaesthesia in healthy animals with controlled modest hypotension, no adverse reaction on renal function was detected (Crandell et al, 2004; Boström et al, 2002). However, in these studies, some dogs developed changes in renal parameters, underlining the importance of maintaining a normotensive state during anaesthesia when considering NSAIDs in the preoperative period.
A summary of NSAIDs available in companion animals is presented in Table 4.
Paracetamol
Paracetamol’s mechanism of action is complex and includes effects on both the peripheral (COX inhibition), and central (COX, serotoninergic descending neuronal pathway, L-arginine/nitric oxide pathway, cannabinoid system) antinociception pathways.
Paracetamol is generally used alongside NSAIDs as part of a multimodal approach, or instead of NSAIDs when these are contraindicated. It has analgesic and antipyretic effects, but little, if any, anti-inflammatory activity.
A licensed formulation of paracetamol for dogs is available, which is a mixture of codeine and paracetamol. However, because in dogs the bioavailability of codeine is low (Kukanich, 2010), this drug does not contribute to the analgesia. Paracetamol should not be given to cats (Mathews, 2010). Doses and indications are included in Table 4.
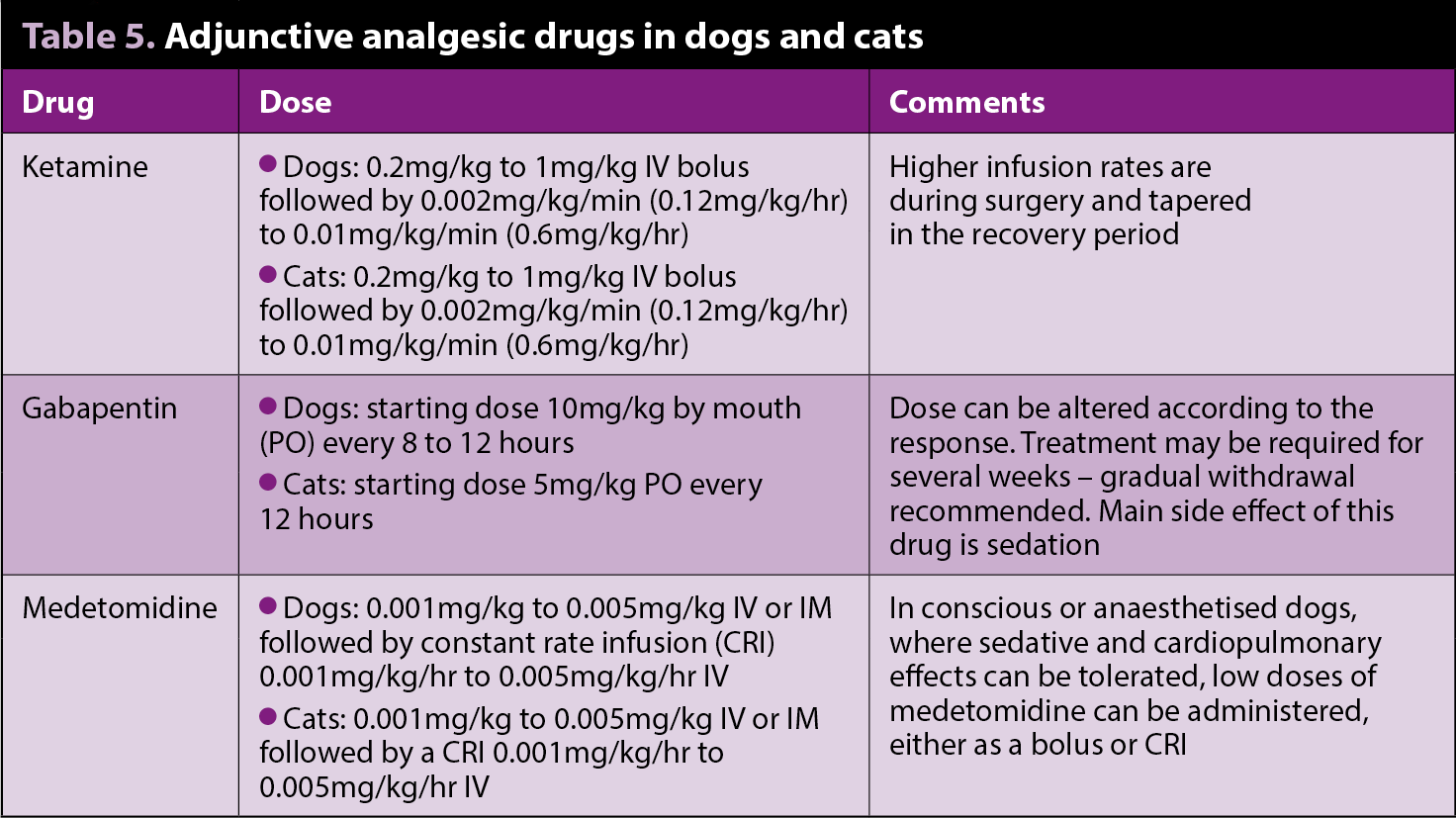
Ketamine
Ketamine is an N-methyl-D-aspartate receptor antagonist that has been increasingly used as part of multimodal analgesia to manage moderate to severe pain in the perioperative period for major surgery. Ketamine is particularly indicated when neuropathic or chronic pain is present.
Studies that evaluate the perioperative analgesic effects of ketamine are limited. Dogs undergoing forelimb amputation receiving a ketamine bolus, followed by CRI at rates similar to those aforementioned, resulted in a significantly lower pain score with respect to a control group, 12 and 18 hours after surgery, although opioid requirements were not significantly different between the groups (Wagner et al, 2002).
Gabapentin
Initially developed as an anti-seizure drug, gabapentin has been used as part of multimodal treatment in humans with neurogenic pain, and perioperatively in laboratory animals with induced nerve damage.
Very few studies have been published assessing its analgesic action in small animals. When given postoperatively in dogs with intervertebral disc surgery, or dogs undergoing forelimb amputation (Wagner et al, 2010; Aghighi et al, 2012), no significant benefit was found from the use of gabapentin at a dose of 10mg/kg every day; however, additional studies with different doses, frequencies and other painful conditions are warranted.
In the long-term treatment for chronic pain in head trauma and musculoskeletal disease in cats unresponsive to traditional analgesic therapy (Lorenz et al, 2013), supporting gabapentin at an average dose of 6.5mg/kg every 12 hours indicated satisfactory pain control.
Alpha-2 agonists
Although alpha-2 agonists are not considered first-line analgesics, however, they are successfully used as adjunctive analgesics in a variety of clinical settings, supplementing analgesia and producing a synergistic analgesic effect when administered with opioids while reducing stress response. The major factor that limits their use in pain management protocols is their significant haemodynamic side effects, which can be reviewed elsewhere (Sinclair, 2003; Murrell and Hellebrekers, 2005).
Recent attention in veterinary medicine has focused on the administration of CRI alpha-2 agonists during the intraoperative and postoperative periods to supplement anaesthesia and analgesia, although little evidence exists evaluating the effectiveness of these infusions in veterinary patients.
Other than systemic administration, alpha-2 agonists may be administered by novel routes. In dogs, incorporating a low dose of medetomidine combined with opioids or local anaesthetics into an epidural protocol produces synergistic analgesic effects (Pacharinsak et al, 2003). In conjunction to local anaesthetics, medetomidine prolonged sensory and motor blockade after radial nerve block in dogs (Lamont and Lemke, 2008) and may prove to be an effective adjunct to local blocks.
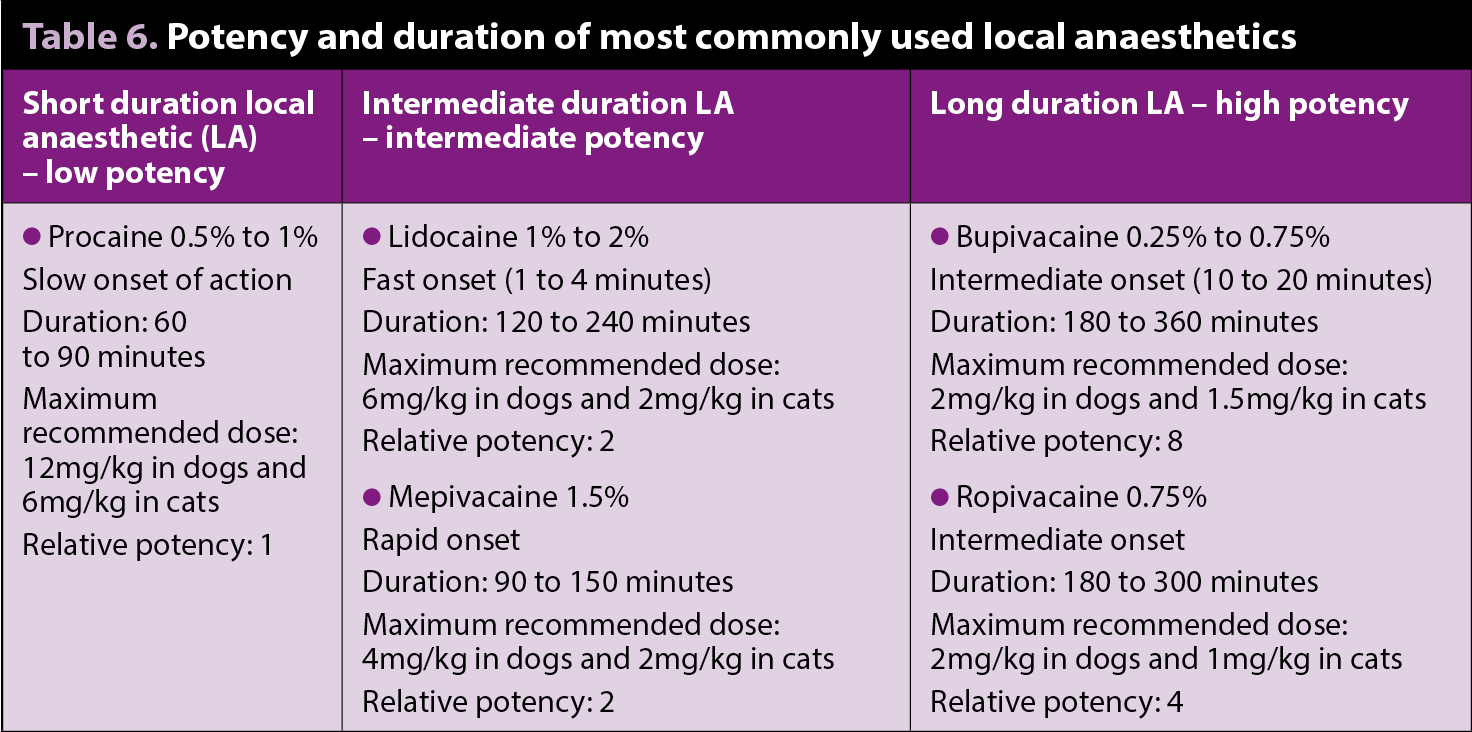
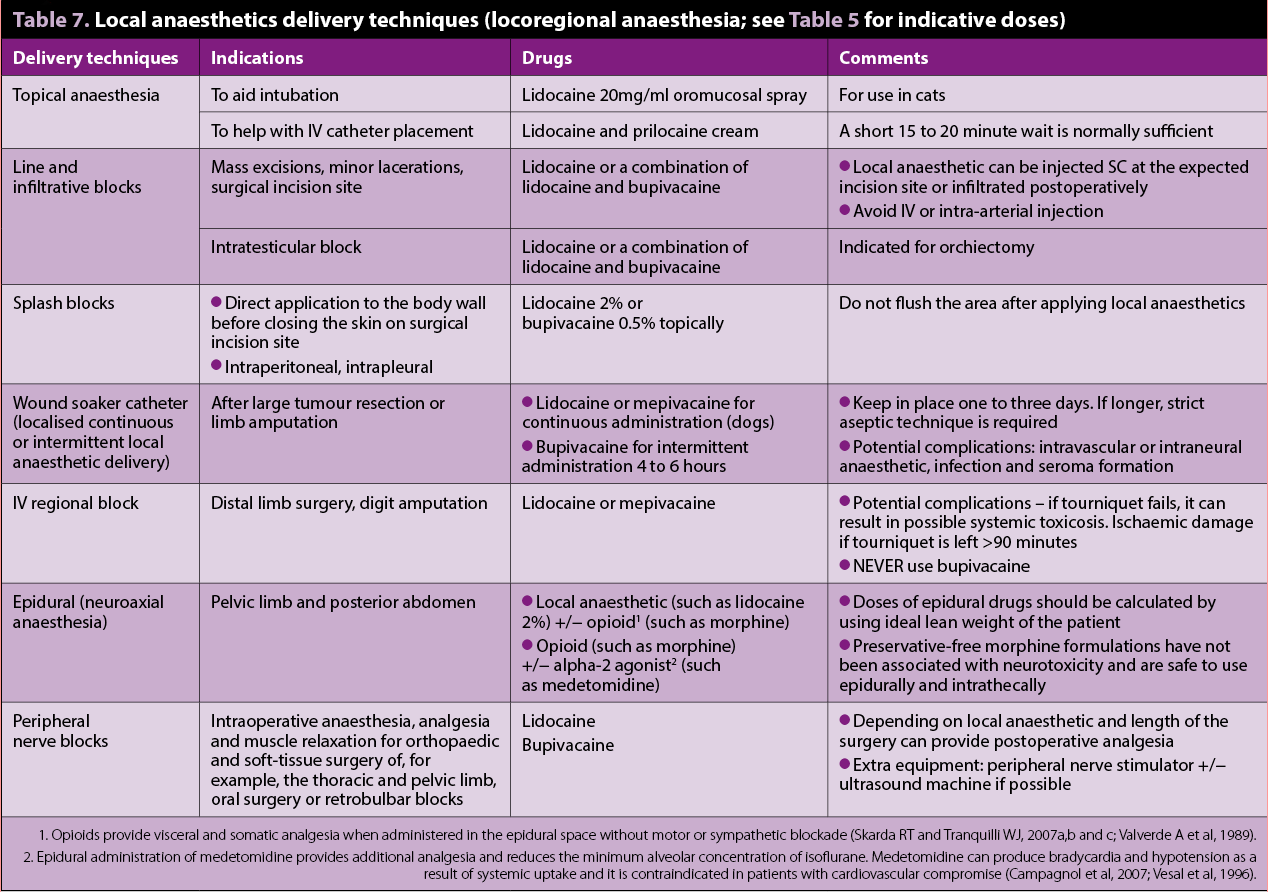
Local anaesthetics
Local anaesthetics are the main drugs used for locoregional anaesthesia and analgesia (Lemke and Dawson, 2000; Figures 2 and 3). Evidence shows local anaesthetics not only have antinociceptive, but also immune-modulating antimicrobial and tissue-healing, effects (Johnson et al, 2008; Cassuto et al, 2006).
Lidocaine can be used IV as a bolus or as a CRI in dogs to provide prokinetic, antiarrhythmic, inhalant-anaesthetic sparing, anti-inflammatory effects and systemic analgesia. It can be combined with methadone (or morphine)
and/or ketamine in a CRI for postoperative analgesia (Figure 4).
A pilot study on the analgesic effects of IV lidocaine in dogs undergoing intraocular surgery (Smith et al, 2004) demonstrated a bolus of 1mg/kg, followed by a CRI of 0.02mg/kg/min produced comparable postoperative analgesia to morphine 0.15mg/kg IV bolus followed by a CRI of 0.1mg/kg/hr. However, other studies on the use of lidocaine infusions in cats (Thomasy et al, 2005; Pypendop et al, 2006) suggest caution should be taken until more data is available. These studies failed to show any beneficial antinociceptive effects in cats with moderate plasma levels of lidocaine. This drug also presents greater toxic effects in cats compared to dogs.
Non-pharmacological pain management post-surgery
The use of non-pharmacological therapy, alongside a good pharmacological treatment as part of a multimodal approach to pain, is becoming more popular and evidence is emerging to support their use.
Cold therapy
Cryotherapy, or cold therapy, is the least expensive, easiest and most effective non-pharmacological method to decrease acute inflammatory pain (such as that induced by surgery, trauma or exercise).
Postoperative orthopaedic, neurological and soft tissue (when appropriate) patients can receive immediate cold therapy on their incisions and surgery sites. Cold therapy immediately after cranial cruciate ligament surgery can significantly decrease pain, swelling and increase the joint’s range of motion. When combined with compression, it has proved to be more effective (Drygas et al, 2011).
Commercial canvas packs or resealable plastic bags of ice wrapped in a towel can be used for 10 to 20 minutes every 6 to 8 hours. A dampened towel allows for a more rapid transfer of heat and quicker cooling of treated tissues (Dragone et al, 2014), as shown in Figure 5. Owners can be instructed to continue cold therapy at home until any swelling and oedema diminishes – usually for three to four days post-surgery, depending on the procedure.
Other approaches
Further methods of non-pharmacological management include the use of physiotherapy, when appropriate, good nursing care or recovering the patient in a comfortable and warm environment.
Conclusions
An appropriate postoperative analgesia regime should prevent upregulation of pain pathways and the development of central and peripheral sensitisation (pre-emptive analgesia), and should be combined with regular pain assessments.
Although opioids and NSAIDs remain the cornerstone for postoperative pain management, the use of adjuncts should always be considered for patients undergoing very painful procedures or where traditional analgesic drugs are insufficient to provide adequate analgesia. Adjunctive drugs can be administered via separate infusion devices to allow adjustment of doses independently.
Non-pharmacological management of postoperative pain (such as physiotherapy and cold therapy) should be applied, when appropriate.
Acknowledgement
The author would like to thank Alessandra Mathis, DVM, CertVA, DipECVAA, MRCVS, for reviewing this article.