4 May 2022
Christopher Scudder BVSc, PhD, DACVIM-SAIM, DECVIM-CA, MRCVS, Eleanor Haskey BSc(Hons), PGCert, VPAC A1, VTS(ECC), RVN and Vicky Maund BSc(Hons), Cert VN ECC, CVN, DipAVN, DipHE CVN, PGCert Vet Ed, VTS(SAIM), RVN review current knowledge around the complications associated with this procedure in feline and canine patients, and make an appeal to the profession to aid their study with the aim of minimising the risks.

Peripheral IV catheters (PIVCs) are placed in many, if not most, veterinary practices on a daily basis. Therefore, PIVC placement and management is likely to be one of the most frequently performed clinical procedures in veterinary medicine.
Several studies and reviews describing PIVC complications in cats and dogs exist; however, large multicentre studies lack robust evidence (Guzmán Ramos et al, 2018; Jones et al, 2009; Mathews et al, 1996; Parkes, 2015; Seguela and Pages, 2011; Tan et al, 2003).
PIVCs are typically placed into a vein of a distal limb and remain in situ for a few minutes up to several days. They have many different characteristics compared to central venous catheters and peripheral inserted catheters in veterinary medicine, being typically shorter in length, typically single lumen and taped instead of sutured in place.
The common complications associated with use of PIVCs are pain during removal, residual tape glue remaining on the patient skin, thrombosis, phlebitis and contamination with either bacteria or fungi. Numerous factors have been associated with complication risk.
This article will review the current knowledge of PIVC use and risk factors for complications in cats and dogs, and highlight opportunities to improve our understanding of how best to manage these devices.
Numerous factors are present at the time of placement of PIVCs that have been associated with risk of a complication.
One risk factor is the role of the personnel placing a PIVC in practice. One UK study reported PIVCs placed by veterinary students had fewer complications, while a study undertaken in Spain described PIVCs placed by students and veterinarians with less than one year since graduation having higher contamination rates compared to more experienced staff (Guzmán Ramos et al, 2018; Parkes 2015).
Good hand hygiene prior to placement of a PIVC has been shown to decrease PIVC-related complication in humans, but continues to be under or incorrectly performed (Hirschmann et al, 2001; Moureau, 2014; Slater et al, 2019; Zingg et al, 2009).
Poor hand hygiene may be a leading cause of PIVC-related complications, and is deemed so important that it is a component of PIVC care bundles in human and veterinary literature (Hancill, 2013; Kleidon et al, 2019; Ray-Barruel et al, 2019).
The methodology of skin preparation might be associated with risk of complication.
Recently, recommendations have been made that skin is disinfected using 2% chlorhexidine rather than povidone-iodine in humans (Timsit et al, 2020). In veterinary medicine, one study reported no difference of contamination when skin was prepped using 2% chlorhexidine, then wiped with 70% isopropyl alcohol versus 70% isopropyl alcohol alone (Guzmán Ramos et al, 2018). Use of 4% chlorhexidine prior to PIVC placement resulted in lower bacterial colonisation and dermatitis compared to no skin preparation in another study (Coolman et al, 1998).
Interestingly, no difference was found of phlebitis between groups with mild phlebitis reported in all veins that had catheters placed, although the PIVC was in situ for longer in the chlorhexidine prepped group.
The authors are not aware of veterinary studies describing PIVC complication rate with povidone versus chlorhexidine skin preparation.
Another factor investigated at the time of PIVC placement has been the number of PIVC insertion attempts, with no difference of PIVC contamination between one or greater than placement at first attempt (Jones et al, 2009).
Other factors associated with PIVC complication reported in human, but not cat or dog, medicine include clipping versus shaving hair, use of antibiotic creams or ointments at PIVC insertion site and PIVC material (Tanner et al, 2011).
Following PIVC placement, the catheter may be used for blood sampling, then sealed using a needleless or non-needleless bung, T-connector or Y-connector.
Both the risk of blood sampling and use of T-connector or Y-connector were assessed by Jones et al (2009). This study reported that T-connectors were associated with a greater risk of bacterial contamination compared to Y-connectors, and that blood sampling did not increase the risk of contamination.
Use of needleless bungs decreased phlebitis incidence in one human study, and the type of needleless bung may also influence complication risk, but this has not been reported in veterinary medicine (Btaiche et al, 2011; Ronen et al, 2017).
After attachment of a connector or bung, PIVCs in veterinary medicine are typically secured using tape and then covered using soft dressing.
The type of tape and dressing and risk of PIVC complication has not been well described in veterinary medicine, but has been associated with PIVC complication risk in some human studies (Ullman et al, 2020).
Factors that need to be taken into consideration include frequency of undressing the catheter and inspecting to insertion site, the adhesive tape used, frequency of flushing the PIVC and whether to routinely flush PIVCs if a CRI is being administered, and what type of fluid to use for flushing.
Frequency of inspection of the cannulated vein and PIVC site ranges from several times to once daily (Davis, 2015; Holgate, 2019; Marsh-Ng et al, 2007; Tan et al, 2003). While increased PIVC manipulation could increase the likelihood for a complication, early identification of a pre-existing complication should decrease any local or system sequelae.
The optimum inspection frequency requires evidence to help with developing any recommendation. Flushing frequency recommendations also vary from every 4 to 8 hours, to every 12 to 24 hours; a lack of evidence currently exists for these guidelines in veterinary medicine (Haskey, 2016; Tan et al, 2003).
When flushing a PIVC, the bung should be disinfected before attachment of the flush solution. Human literature reports disinfection with 70% alcohol and scrubbing for 5 to 15 seconds, or use of alcohol disinfection caps, or chlorhexidine-alcohol scrubbing for longer residual anti-bacterial decontamination (Devrim et al, 2019; Hong et al, 2013; Moureau and Flynn, 2015).
It seems logical to extrapolate these recommendations to veterinary medicine, although specific veterinary studies are currently not available.
The solution used to flush a PIVC may also vary from institute to institute. Flushing using 2 international units (IU) or 10IU heparin sodium in 0.9% saline or plain 0.9% sodium chloride resulted in equivalent PIVC failure rates and length of time the PIVC remained patent, although flushing with heparin may increase the likelihood of using the PIVC for blood draws (Silver et al, 2017; Ueda et al, 2013).
No difference was reported of time to occlusion of pedal arterial catheters in dogs when using either 4IU/ml heparin saline in 0.9% sodium chloride or 0.9% sodium chloride flushing. No difference was also reported of the number of central catheters still patent at 72 hours post-placement in dogs when using 10IU/ml heparin sodium in 0.9% sodium chloride or 0.9% sodium chloride flush (Sasaki et al, 2020; Vose et al, 2019).
At least some evidence exists for the recommendation that either heparin sodium between 4IU/ml to 10IU/ml diluted in 0.9% sodium chloride or plain 0.9% sodium chloride is suitable to maintain PIVC patency.
The removal of a PIVC may be premature due to patient interference, the development of a complication or planned due to the PIVC being in situ for a defined period of time and intentionally removed to decrease the likelihood of PIVC complication developing.
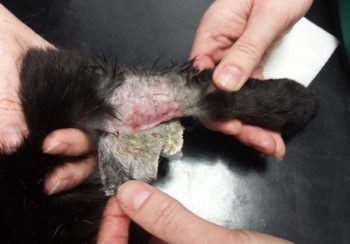
The recommendations when to routinely remove a PIVC vary in both human and veterinary medicine, and no consensus appears to exist in human medicine whether to remove a PIVC at a defined time or only at the appearance of clinical signs of phlebitis, an infection or catheter failure (O’Grady et al, 2011).
The risk of PIVC bacterial colonisation in dogs increased with time in situ in two studies, with 40% of PIVCs being contaminated after 72 hours in one of these; this increased contamination after more than 72 hours was not observed in two other studies (Guzmán Ramos et al, 2018; Marsh-Ng et al, 2007; Mathews et al, 1996; Seguela and Pages, 2011).
The risk of phlebitis may increase with increasing dwell time of more than 72 hours (Mathews et al, 1996; Parkes, 2015). Therefore, veterinary medicine seems to parallel human medicine, with the strength of evidence of routine removal of PIVCs at a defined time being weak, but if implemented, then being after 72 to 86 hours post-placement.
Factors not discussed previously that may also influence this risk include the drugs and blood products administered via the PIVC, and patient factors such as location of PIVC placement and their health status.
More evidence is needed – particularly in cats – to help develop best practice guidelines. The authors are, therefore, launching a multicentre study regarding PIVCs in the UK in early 2022.
This will be a two-phase study; phase one will be a large multicentre clinical audit and this data will then be used to identify risk factors for PIVC complications. These factors will be used to develop a care bundle aiming to minimise PIVC complications, and phase two will be implementation of the care bundle and assessment of whether this reduces PIVC complications.
The authors would like to recruit as many practices as possible into this study.
If you would like more information or would like to be involved in this study then please email [email protected]