17 Jul 2017
Karen Perry looks at detecting and testing for joint pain in small animals using an organised and systematic approach.

Joint pain is commonly encountered in small animal patients, with approximately 20% of the canine population reported to suffer chronic pain associated with OA. Accurate detection of pain is critically important to allow treatment protocols to be instigated, monitored and adjusted appropriately. However, pain is a subjective sensation and its detection can be difficult. This article details a systematic approach to the diagnosis of painful joints. History-taking forms an important part of this and may be facilitated by the use of validated, owner-completed questionnaires available for both dogs and cats.
General observation and gait assessment, followed by a detailed orthopaedic examination, allow localisation of the cause of pain in most cases. Further diagnostic tests can then be selected as appropriate, including radiography, ultrasonography, advanced imaging and synoviocentesis. Occasionally, cases will be encountered where an initial examination does not allow localisation of the source of pain. In these challenging scenarios, additional tests may be required, potentially including a full neurological examination, intra-articular anaesthesia and nuclear scintigraphy.
Joint pain is commonly encountered in veterinary patients, with approximately 20% of the canine population reported to suffer chronic pain associated with OA (Pfizer, 1996).
Accurate detection of pain, however, is difficult. Pain is a subjective sensation and, therefore, should be assessed by the affected individual. Clearly, our veterinary patients cannot directly provide us with this information; therefore, we must use other methods.
As clinicians, it is important we develop an organised and systematic method for evaluating joint pain that can be repeated with minimal variation. This reduces the likelihood a particular aspect of the examination will be overlooked. While some variation will be seen between different clinicians in how they approach a case, essential components include taking a patient history, general observation of the animal, gait assessment and a more focused orthopaedic examination. In most cases, these steps will allow the source of discomfort to be localised, but further diagnostic procedures will often be required to confirm or further specify a diagnosis.
The most commonly used diagnostic tools for the orthopaedic work-up are diagnostic imaging, cytological and bacteriological examination of synovial fluid, and cytological and histopathological interpretation of masses and bone lesions. When the source of lameness cannot be detected during an orthopaedic examination, reaching a definitive diagnosis can be substantially more challenging and different diagnostic techniques may be required.
Obtaining sufficient background information is critical to interpreting the orthopaedic examination. The importance of history-taking is often underestimated; however, some activities are difficult to assess in the clinic. Signs such as lameness, ability to jump, and ability to ascend and descend stairs can be difficult to assess in a clinical situation as some dogs will not jump or attempt stair climbing at the clinic, while other dogs will jump as they never would at home; thus a vet can easily misinterpret these findings.
Although owner evaluations are subject to the placebo effect, owners are able to offer insight into their pet’s behaviours in a natural setting (Brown et al, 2008). To obtain this information, a careful history should be taken, or you may find it helpful to use a client questionnaire in conjunction with your clinical examination findings.
The first item of information to gather is usually the client’s primary concern. It is important to ensure the client is communicating correctly; simple observations, such as which leg the animal is lame on, may be incorrect if the client thinks of the leg while the animal is facing him or her, rather than considering from the animal’s viewpoint. A detailed history of the problem should be obtained, including chronicity, manner of onset, any inciting incident, severity and character of the lameness, association with rest or activity, progression, presence in other limbs, previous treatments, and the perceived efficacy of these and any associated systemic signs.
When an owner presents a pet for evaluation with a concern it may be suffering from chronic joint pain, a number of behaviours are reported that owners attribute to the painful condition (for example, it no longer climbs stairs or jumps on to the bed; Brown et al, 2007). Answers to certain questions have been shown to be significantly different between dogs suffering with joint pain and those without painful joints (Hielm-Björkman et al, 2003) and may be particularly useful to include in the history-taking. These include questions regarding:
In addition to the questions above, night-time restlessness has been postulated to be associated with joint pain in dogs (Knazovicky et al, 2015) and, therefore, questioning owners about this is recommended.
The history may not be as helpful for cats as it is for dogs. Mildly injured cats often do not exhibit obvious or specific clinical signs and the onset of any clinical signs can be gradual – making them even more difficult to appreciate. Outdoor cats spend a lot of time unobserved by their owners, with general habits including urination, defaecation and grooming often not seen for days at a time. Overt lameness is quite uncommon in this species (Clarke et al, 2005) and, therefore, history-taking should include questions regarding other behaviours.
Presentations associated with joint disease and discomfort have included stiffness, a shuffling gait, difficulty jumping, weakness and inactivity (Godfrey, 2005). Other presenting complaints have included reduced grooming, altered temperament (Bennett and Morton, 2009) and inappropriate elimination habits (Slingerland et al, 2011).
As clinicians, we may have a tendency to concentrate inappropriately on questions regarding decreased activity levels in cats (Benito et al, 2012). Inactive behaviours are also important to a cat’s quality of life and joint pain can lead to changes in these, such as an inability to sleep or rest normally or being less comfortable while petted. When taking a history regarding orthopaedic disease, any effect on these inactive behaviours should also be investigated.
Client questionnaires allow the person most familiar with the pet to provide insight on its abilities in the home environment. The client-specific outcome measure is a questionnaire for evaluating OA pain by assessing the daily activities and specific activity impairments for an individual pet as identified by the owner. Examples may include playing with other animals, difficulty moving after rest, difficulty moving after major activity, trotting, urination, getting in and out of a car, and jumping on to furniture. Owners specifically indicate places and times when they observed impaired activities, then rate the degree of impairment. This method has demonstrated owners are able to assess differences in their pet’s mobility when an analgesic is administered compared to a placebo (Lascelles et al, 2008).
Another veterinary-developed owner questionnaire is the canine brief pain inventory (CBPI). Developed to measure OA pain, it has also been used to measure cancer pain in dogs (Brown et al, 2007; 2008; 2009). The CBPI is based on 10 questions aimed at quantifying the severity of pain and how it interferes with the dog’s normal activities. It has been shown to correlate with force-platform data (Walton et al, 2013).
Other owner-completed questionnaires include the Liverpool OA in dogs clinical metrology instrument (Hercock et al, 2009), the Helsinki chronic pain index (Hielm-Björkman et al, 2003; 2009) and the University of Glasgow veterinary school questionnaire (Wiseman-Orr et al, 2004; 2006). These four questionnaires are all appropriate for use in dogs. For cats, the feline musculoskeletal pain index is a subjective owner-completed instrument developed to assess chronic feline degenerative joint disease-associated pain (Benito et al, 2013).
At some point – potentially while taking the history – an opportunity should be taken to observe the animal while it is relaxed and moving. Particular attention should be paid to how the animal sits or stands. For example, animals with stifle pain are generally reluctant to fully flex the stifle joint and may sit with the affected limb out to the side.
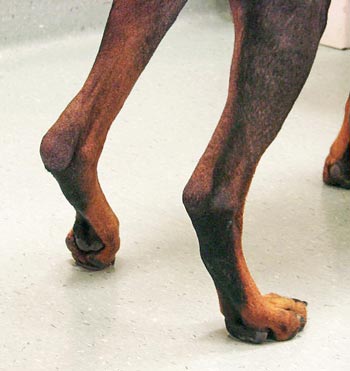
Similarly, most animals stand with their sound limbs further under the body – allowing them to take a greater proportion of weight, while placing a lame limb in a more abducted position. Other aspects of stance that may be noted at this time include the presence of a palmigrade or plantigrade stance (Figure 1), but these abnormalities may only be detected when the animal is bearing weight or when it is walking or trotting (Renberg, 2001).
During gait assessment it is helpful to watch the animal moving at various speeds. The main objective of this part of the examination is to localise the lesion to a particular limb and determine if the lameness is more likely orthopaedic or neurological in origin. Animals experiencing pain in a limb may attempt to transfer weight off it and ambulate with the affected limb in contact with the ground for the shortest possible time.
In the case of thoracic limb lameness, animals typically raise their head while the affected limb is in contact with the ground and lower their head when the sound limb is in contact with the ground. This gait gives them the classic “head-bob” appearance as they walk or trot and is a reflection of them transferring weight away from the painful limb. In the case of pelvic limb lameness, a “head-bob” will reverse; the head will be raised when the unaffected limb is on the ground, but this can be difficult to detect (Renberg, 2001).
Cats with pelvic limb lameness may demonstrate a hip hike or a tail shift where the tail moves away from the lame limb during weight-bearing. Subtle gait abnormalities can be challenging to detect, particularly in smaller dogs. Taking videos and playing back in slow motion can help here, and specific programs are available that facilitate this where videos can be played in slow motion and annotated.
Feline gait assessment can be difficult and time-consuming as most cats will not walk on a lead, and often refuse to move around in a new environment. Thus, it can be helpful to:
After observing the animal at rest and moving, the clinician should be able to tell which limb(s) seem to be the most affected. The next phase of examination is to gently palpate the animal while it is standing in a relaxed manner. At this point in the examination the clinician should be focusing on asymmetry. By gently palpating up and down the right and left limbs simultaneously, the clinician can note atrophy or swelling and joint effusion most readily. Comparing the contralateral limb helps to determine the significance of a finding unless the lesion is bilateral.

While the animal is standing quietly, the clinician should also check for the presence of proprioceptive deficits as a minimal test to eliminate neurological disease (Renberg, 2001). Weight-bearing asymmetry can also be assessed with the patient standing. For the pelvic limbs, gently pulling back caudally with two fingers on the metatarsus will cause the animal to unload the limb. If this is compared between sides, the resistance to unloading will be greater on the unaffected side.
Similarly, for the thoracic limbs, this can be achieved by applying cranial pressure in the region of the accessory carpal bone, which will cause the animal to “knuckle” the limb over (Voss and Steffen, 2009).
In the past, studies designed to evaluate pain in dogs relied heavily on a vet’s assessment of lameness supported by values generated through gait analysis by use of a force plate. When collected properly, data on gait analysis can offer an objective measure that can be reliably monitored over time; however, it can be extremely time-consuming, requires specialised equipment and relatively strict inclusion criteria.
This form of gait analysis is unlikely to be available or practical in most practices. In addition, these measures only evaluate an animal at one specific point in time and weight-bearing on an affected limb is only one part of the much larger picture of chronic pain in dogs with joint disease (Brown et al, 2007).
Following gait assessment, a more focused inspection is necessary to localise the lesion more specifically. The orthopaedic examination should be performed with the animal in a comfortable position. For some patients, this is best performed with the animal placed in lateral recumbency. Other patients are reluctant to lay in lateral recumbency, however, and, for these, the thoracic limbs are best examined with the animal sitting and the pelvic limbs with the animal standing. A systematic evaluation of each limb is begun. The clinician should generally start with the unaffected limbs and conclude with the limb most likely to be painful. This minimises the risk of the animal becoming intolerant to examination after the painful focus is located.
Additionally, starting at the toes and working proximally up the limb reduces the chance of confusing the source of pain – for example, when the hips are extended looking for hip pain, the stifles are also extended in the process. If the stifles have not already been eliminated as a source of discomfort, any pain elicited on hip extension could be just as reasonably attributed to either joint.
This article focuses on abnormal findings associated with the joints themselves, but a full orthopaedic examination should also evaluate the paw pads, interdigital spaces and long bones. When palpating a joint, the clinician should gently cycle it through a range of motion to note any crepitus and increases or decreases. The joint should then be placed at the limit of its range of motion. At that point, additional gentle pressure should not meet with discomfort (Renberg, 2001).
The joints of the phalanges should be assessed for pain, crepitus, swelling and instability. Instability is easiest to evaluate with the joints held in extension, as they may be falsely perceived to be lax if examined in flexion. Special attention should be paid to palpating the sesamoids on the plantar and palmar aspects of the metatarsophalangeal and metacarpophalangeal joints. Be aware many dogs and cats resent palpation or manipulation of their feet, even when having no pain or injury in that area. If examination of all four paws is poorly tolerated this may be a behavioural problem rather than representative of pain.
The hock should be assessed for pain, effusion, swelling and instability. Pain in the hock is most easily elicited by placing the joint in extension. A normal animal should not resent 180° of extension (Renberg, 2001). Effusion in the hock can most reliably be felt on either side of the calcaneus just caudal to the malleolus. A lack of crisp outlines or soft tissue distension here is likely caused by effusion within the tibiotarsal joint. With the joint in moderate extension, the examiner should exert medial, lateral, cranial and caudal pressure on the foot in an attempt to demonstrate laxity. Animals previously noted to have a plantigrade stance should be examined for the presence of tarsal luxation or calcaneal tendon tears.

With the stifle held in extension, an attempt to flex the hock should be made. A normal hock does not flex with the stifle extended and any abnormality suggests a disruption at some point of the muscle-tendon unit. If the hock flexes, it is important to note if the digits also flex, which may indicate the digital flexors are intact (Renberg, 2001). If the lesion in an animal with a plantigrade stance does not seem to involve the calcaneal tendon, attempts should be made to manipulate the intertarsal and tarsometatarsal joints to investigate any luxation or subluxation there.
To test for the presence of pain in the stifle, the clinician should place the joint in to full extension. Evidence of medial or lateral instability should be noted. Effusion in the stifle is best detected by palpating the edges of the straight patellar ligament with the joint in moderate flexion. The ligament should be easily palpable, and effusion is felt as a softness or puffiness on either side of the ligament – preventing crisp borders from being felt.
The clinician should also check for the presence of a firm swelling on the medial aspect of the stifle – the so-called medial buttress (Figure 2). This is composed of fibrous tissue and is typically present in cases with cranial cruciate ligament disease. The stifle should be evaluated for laxity in cranial drawer and cranial tibial thrust. Even if no laxity is detected, if the index of suspicion for cranial cruciate ligament disease remains high, the patient should be sedated and re-examined. Some animals are able to guard their stifle to the extent they can mask any drawer motion present (Renberg, 2001).
The clinician should also attempt to luxate the patella. This should be attempted by exerting pressure against the side of the patella and attempting to dislodge it from the trochlear groove. This manoeuvre should be attempted in flexion and extension as well as in medial and lateral directions.
Pain is most consistently noted in the coxofemoral joints when the hips are extended (Renberg, 2001), but this can also occur during abduction or flexion (Impellizeri et al, 2000). Animals with lower spinal pain may also resent extension of their hips, thus this possibility must be carefully investigated. A normal animal does not resent having its hips extended to almost 180° (Figure 3). Evidence of coxofemoral subluxation can be noted through a variety of tests, but the most reliable is Ortolani’s sign. Most dogs resent this test and it is best performed under sedation.
A variety of methods can determine if the hip has luxated. Most luxations are craniodorsal in nature and can be readily detected, although final confirmation requires radiographs. The “thumb test” involves placing the thumb in the patient’s ischiatic notch just caudal to the greater trochanter (Renberg, 2001). The limb is then externally rotated, which results in the examiner’s thumb being squeezed or displaced by the greater trochanter if the joint is in place. In a dog with a craniodorsal luxation, the external rotation causes the femoral head to rotate cranially and the greater trochanter does not move. The examiner’s thumb is, therefore, not squeezed or displaced (Renberg, 2001).
Alternatively, the examiner can note the relation between the ischiatic tuberosity, the greater trochanter and the crest of the ilium. These three landmarks should form an obvious triangle, with the greater trochanter forming a downward point (Figure 4). In the case of a craniodorsal luxation, the three landmarks form more of a line. Ventral luxations are less obviously palpated, but the relation between the three landmarks is different than that seen on the contralateral side with the greater trochanter being more medially located. The femoral head can also sometimes be palpated per rectum.
When the carpus is examined, the investigator should palpate the cranial aspect of the joint, with the joint held in mild flexion. The carpal joints should be easily palpable on the cranial surface with no puffiness. An inability to palpate the individual joints over the cranial surface is likely caused by joint effusion. The carpus is best evaluated for pain by placing it in flexion. A normal joint can be flexed to the extent the digital pads touch the antebrachium without pain.

The carpus should also be evaluated for medial and lateral laxity, and hyperextension. Normal animals should have minimal extension of the carpus past 180°. If the animal was noted to have a palmigrade stance, attempts should be made to verify the abnormality by firmly extending the joint.
When the elbow is palpated, it should be assessed for medial and lateral laxity, as well as for range of motion and effusion. Effusion in the elbow is best palpated medial and lateral to the olecranon. The relation between the landmarks of the elbow in a normal dog should be noted because luxations are often diagnosed by palpating an excessively prominent medial epicondyle and not being able to palpate a distinct lateral epicondyle (assuming a lateral luxation; Renberg 2001).
Similarly, condylar fractures are commonly diagnosed by noting an abnormal relationship between the medial and lateral epicondyles. The normal elbow should be able to be flexed to the point that the muscles of the antebrachium meet the muscles of the upper limb and be able to be extended to almost 180° (Figure 5). Pain is best noted by applying firm pressure in extension or by pronating and supinating the elbow while it is held in moderate flexion. Firm pressure should be applied over the region of the medial coronoid process of the ulna, as a pain response here is sometimes the only abnormality in dogs with medial coronoid process disease.
At the shoulder, the clinician should carefully place the joint through a range of motion. Normal dogs and cats should tolerate having the joint flexed and extended parallel to the spine (Figure 6) and abducted approximately 30°. Pain can be elicited in flexion, extension or abduction. Often, mild internal rotation with flexion elicits a painful response in dogs with pathology of the shoulder (Renberg, 2001).
It can be difficult to separate pain in the elbow from pain in the shoulder, as pressure is often exerted on one joint while examining the other. The clinician should be especially diligent in trying to differentiate pain in one joint from the other, but isolating the source of discomfort may not be possible on physical examination alone. When an investigator is suspicious of bicipital tenosynovitis, the shoulder should be flexed while the elbow is extended, so as to stretch the entire myotendinous unit. Applying digital pressure over the biceps tendon of origin may be necessary to elicit this discomfort (Figure 7). The relationship between the acromion and greater tubercle should also be assessed to evaluate for luxation. Luxations of the shoulder can be medial or lateral, and may reduce and reluxate easily. To check for this, the clinician should palpate the joint lightly while placing it through a range of motion (Renberg, 2001).
The clinician should also evaluate for spinal pain. This should be performed in every animal – even if no evidence of neurological injury is noted – as root signature may be the only presenting clinical sign of a neurological lesion. Firm dorsal pressure can be exerted over the spine without causing discomfort in the normal dog. Because spinal hyperpathia can be severe in affected dogs, it is advisable to begin with gentle palpation and work up to firm pressure – stopping when discomfort is noted.
Additionally, the range of motion of the neck in all directions should be assessed. Most dogs can touch their nose to their ribs. Caution should be exercised if there is any suspicion of spinal fracture or other instabilities (Renberg, 2001). Be aware, many cats react to palpation along the lower back and adverse reactions here should be interpreted with caution as they are often clinically insignificant.
Once the likely source of lameness has been ascertained, through history-taking, gait assessment and orthopaedic examination, it is likely further diagnostics will be required to characterise the lameness and enable a therapeutic plan to be devised.
Radiography is the most commonly pursued diagnostic test when evaluating painful joints and can certainly be very effective. Knowledge of normal radiographic anatomy in the immature and adult animal is crucial. Radiographs of the contralateral limb are useful for comparison – particularly in skeletally immature patients.
Although commonly used, radiography does have some limitations it is important to be aware of. While soft tissue swelling is a consistent and reliable indicator of joint disease or injury, other radiographic changes can take time to become apparent (Ohlerth et al, 2009) – for example:
Therefore, despite a set of apparently normal radiographs taken early in a disease process, a further set of radiographs may be warranted a few weeks later to confirm absence of salient findings.

Ultrasonography is not commonly used for the investigation of joint pain, but can be an accessible and cost-effective way to examine periarticular soft tissues. Muscular diseases, abscesses, haematomas, seromas, lipomas, cellulites and neoplasia may be assessed, and foreign bodies in soft tissue can also be identified. The Achilles tendon (Kramer et al, 2001) and the biceps tendon (Scharf et al, 2004) can also both be imaged using ultrasonography. Additionally, the surfaces of bones can be assessed for discrete changes, including irregularities, discontinuities, periosteal reactions, fragments and sequestrae.
CT, as a cross-sectional imaging technique, can be useful in avoiding superimposition of adjacent structures and is particularly useful in imaging the elbow. CT is more sensitive than radiography for detecting bone lysis and, therefore, is particularly useful for assessment of neoplastic or infectious bone diseases where radiographic changes are not yet evident.
MRI, like CT, is a cross-sectional imaging technique, and so avoids superimposition of structures. It provides excellent contrast resolution and is, therefore, the technique of choice for imaging soft tissues, but provides less bone detail than CT. Unfortunately, MRI of the appendicular skeleton in cats and small dogs is limited due to their small size, but it is frequently used in cases with disease of the spine or brain.
Collection and evaluation of synovial fluid is an essential requirement in the diagnosis of arthropathies. The type and number of leukocytes in the fluid can be used to differentiate between disease conditions. Inflammatory joint disease is probably significantly underdiagnosed due to reluctance by vets to perform arthrocentesis (Lemetayer and Tayor, 2014).
Synovial fluid can be obtained from all major joints of the limbs, even the metacarpophalangeal and interphalangeal joints in cats. Once fluid has been obtained, slides should be prepared immediately and any remaining fluid placed in an ethylenediaminetetraacetic acid tube. A sample for bacteriological culture can also be taken and cultured for aerobic and anaerobic growth, if clinically indicated.
Synovial biopsy and histopathological examination are indicated if synovial fluid cannot be obtained, if the cytological examination of the synovial fluid is not diagnostic or if a tumour is suspected (Ohlerth et al, 2009). Synovial biopsies can be useful in the work-up of immune-mediated arthropathies, rheumatoid arthritis and infectious causes of arthritis.
Bone biopsies are indicated in the presence of lytic and/or proliferative bone lesions. Histopathological examination of the specimen usually allows differentiation between inflammatory and neoplastic processes, and diagnosis of tumour type, which is useful when determining appropriate treatment plans and associated prognoses. Jamshidi needle biopsies have an accuracy rate of 92% for detecting neoplasia versus other disorders and an accuracy rate of 82% for correctly diagnosing tumour subtype (Powers et al, 1988).
Following appropriate diagnostic tests – guided by your history and examination – localisation of the cause of lameness, and at least a provisional diagnosis, should be possible. This can then be used to devise a therapeutic plan and to discuss prognosis with the owners.

In some cases, it is difficult to determine the exact localisation of lameness because of the absence of palpable changes or because of unreliable pain responses due to high pain tolerance, stress, hyperaesthesia or patient aggression (Samoy et al, 2005). While the use of expensive and invasive advanced techniques, including arthroscopy, CT, MRI and exploratory surgery, have received considerable interest in the literature, when working-up a non-localisable lameness it is advisable to employ minimally invasive diagnostic techniques first.
The source of the lameness can be located using non-invasive techniques at least 80% of the time – even with challenging thoracic limb cases (Cook and Cook, 2009). Resorting to advanced imaging of the entire thoracic or pelvic limb in an attempt to locate the source of lameness can often be disappointing as it is not unusual for multiple abnormalities to be present in one limb and the clinical significance of each cannot be determined based on imaging alone.
While lameness is more commonly of orthopaedic origin, neurological causes should always remain a diagnostic consideration, particularly when an initial orthopaedic examination is not rewarding. Distinguishing between orthopaedic and neurological causes of lameness can be difficult, but is critical for an appropriate diagnostic and therapeutic plan to be formulated. In cases where the lesion cannot be localised on examination, a full neurological examination should be carried out, including observation, palpation, postural reactions, spinal reflexes, cranial nerves and sensation testing.
In horses and humans, intra-articular administration of local anaesthetic is commonly used for diagnostic purposes because of the simplicity, safety and low cost of the technique. The principle is simple – local anaesthetic is injected into the joint, the anaesthetic is absorbed and sensation in the area is temporarily blocked. Intra-articular anaesthesia can temporarily resolve lameness caused by a variety of lesions, including synovitis, bone and cartilage fragments, intra-articular ligament damage and eroded cartilage (King, 2005).
Intra-articular anaesthesia can also be used to localise lameness in dogs (van Vynckt et al, 2010) and specifically has been shown to be of assistance in the diagnosis of lameness secondary to medial coronoid process disease (van Vynckt et al, 2012).
Bone scintigraphy is an extremely sensitive tool for detecting changes in bone metabolism associated with skeletal diseases, injuries and arthropathies (Lamb, 1987; 1991; Balogh et al, 1999). Technetium-labelled diphosphonates are used as bone radionuclides. Following administration, technetium-labelled diphosphonates are incorporated into bone mineral and accurately reflect the status of bone remodeling, as well as tissue perfusion. The radiopharmaceutical is injected IV and images can be acquired from two hours after injection (Samoy et al, 2008).
Scintigraphy has been found to be helpful in identifying the localisation of lameness in dogs (Schwarz et al, 2004). Scintigraphy can show marked changes in cases where clinical and radiographic signs were absent or equivocal. It should be noted, however, while bone scintigraphy can localise lameness, the exact nature of pathological uptake cannot be determined and, following lameness localisation, further structural imaging, such as radiography, CT or arthroscopy, will be indicated to further characterise the disease process.
Spotting the signs of joint pain can be challenging in small animals, but, by maintaining a systematic approach, clinicians can maximise their chances of success. A detailed and targeted history, potentially including the use of a validated owner-completed questionnaire, is vital if the findings from physical examinations are to be interpreted appropriately. This should be followed by a general observation of the animal, gait assessment and a focused orthopaedic examination.
In most cases, this will allow the source of the lameness to be localised, then further diagnostic tests can be selected as appropriate to achieve a definitive diagnosis. In cases where the source of the lameness cannot be localised following orthopaedic examination, additional diagnostics may be required – potentially including a neurological examination, intra-articular anaesthesia and nuclear scintigraphy.