31 Dec 2019
Elizabeth Villiers BVSc, DipECVCP, FRCPath, CertSAM, CertVR, MRCVS and Jade Pallett BSc discuss the steps taken when carrying out susceptibility testing to determine appropriate antibiotic therapy in pets.

In an era of escalating antibiotic resistance, it is becoming increasingly important that we accurately identify bacteria and determine the most appropriate antibiotic therapy based on susceptibility testing.
To obtain accurate results, it is important to follow strict guidelines set out by regulatory bodies such as the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST). These guidelines provide details of all aspects of culture and sensitivity testing – from the types of media, concentration of organisms applied to culture plates, and temperature and duration of incubation, to choice of antibiotics that should be tested and how to interpret susceptibility testing.
The CLSI guidelines (2018) include a section on susceptibility testing in animals that is available to purchase. EUCAST does not, as yet, have veterinary specific guidelines, although these are under development by a subcommittee called VetCAST. EUCAST guidelines (2019a) are freely available online.
Ideally, samples should be taken early in the course of infection and before antibiotic therapy has started. If this is not possible, waiting three to five days after therapy has stopped is recommended. Skin samples and samples from mucosal surfaces, such as the ear canal or conjunctiva, are taken using swabs with transport medium such as charcoal Amies or an eSwab that contains Amies medium (see further on). If a skin pustule is being sampled, the surface should be disinfected before obtaining the sample. The pustule is aspirated with a sterile needle and syringe, and is absorbed on to a swab.
Samples from suspected anaerobic infections require special handling. Situations where anaerobic infection may be present include pyothorax, peritonitis, cellulitis, abscesses, bacteraemia, surgical infections and wounds. In these situations, it is preferable to submit fluid or tissue instead of swabs, or use eSwabs (see further on). Standard culture swabs are not ideal for anaerobes because cotton fibres may be detrimental, but if swabs are being used, the swab should be immersed into the transport medium tube as soon as possible to minimise exposure to air.
For fluid and tissue samples, a larger volume of sample will help preserve anaerobic conditions (for example, greater than 2ml of fluid, or sufficient fluid to fill the sample tube, or greater than 2cm2 of tissue wrapped in a saline-soaked gauze swab). Ideally, fluid or tissue samples can be placed in tubes containing anaerobic transport medium – a semisolid agar with reducing agents to maintain anaerobic conditions. It also contains a chemical indicator that turns purple in the presence of oxygen.
An alternative to traditional bacteriology swabs is the eSwab, comprising of a nylon-flocked swab with a pre-moulded breakpoint and a screw cap tube filled with 1ml of modified liquid Amies culture medium. After sampling, the swab is placed in the tube and the swab handle snapped off at the breakpoint before applying the screw cap. The sample elutes from the swab into the liquid medium, which can then be directly applied to culture plates. eSwabs are suitable for both aerobes and anaerobes, as well as fungi and yeasts.
Urine samples obtained by cystocentesis should be taken into plain sterile tubes, which give a higher rate of positive cultures than boric samples, although boric acid tubes are used for voided samples to prevent overgrowth of contaminants. Samples should be kept in a cool place but not refrigerated.
Colombia blood agar
Non-selective general growth medium, produces clear zones of haemolysis for haemolytic bacteria.
Chocolate blood agar
Non-selective enriched medium supporting growth of fastidious organisms such as Neisseria and Haemophilus species.
Cystine-lactose-electrolyte-deficient agar
A differential culture medium. Lactose-fermenting bacteria change medium colour to yellow. Electrolyte deficient to prevent the swarming of Proteus species.
Colistin nalidixic agar
A selective medium for the growth of staphylococci and streptococci.
Brilliance urinary tract infection agar
A chromogenic medium for the presumptive identification of bacteria causing urinary tract infection.
Xylose lysine deoxycholate agar
A differential growth medium used for the isolation of Salmonella and Shigella species from faecal samples.
Campylobacter agar
A differential medium for the isolation of Campylobacter species from faecal samples. Incubated at 42°C to inhibit growth of irrelevant pathogens.
Sabouraud dextrose agar
For dermatophytes, other fungi and yeasts. Acidic pH inhibits bacterial growth.
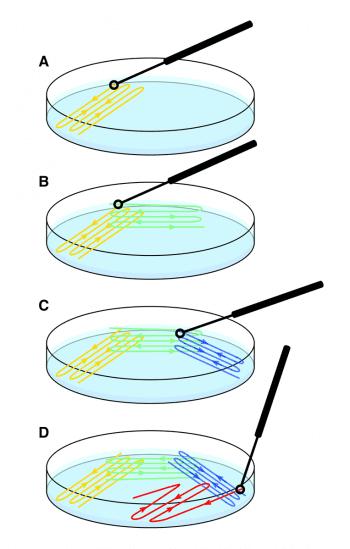
Swabs or samples from fluid harvested into an inoculum loop or samples of tissue are plated out on to various agar plates using a streak technique, which produces individual colonies that can be scooped off for analysis (Figure 1). The sample site determines the choice of agar plates (Panel 1).
Blood agar is a general purpose agar that shows different patterns of haemolysis (see further on). Chocolate agar contains lysed red cells and is used for culture of fastidious respiratory pathogens. Selective agars contain inhibitors such as antibiotics or chemicals that inhibit growth of all or most types of bacteria other than the target organism.
For example, MacConkey agar is selective for Gram-negative bacteria, and contains crystal violet and bile salts that inhibit Gram-positive bacteria, and also lactose, which lead to a colour change in the presence of lactose-fermenting Gram-negative bacteria. Colistin naladixic acid agar is selective for Gram-positive organisms, containing colistin and nalaxidic acid, which inhibit Gram-negatives.
Differential media are used for Salmonella and Campylobacter cultures. The plates are incubated for 24 hours (48 hours for Campylobacter), generally at 37°C in room air, or for Campylobacter, at 42°C in microaerophilic conditions (6% oxygen, 10% carbon dioxide [CO2]). Anaerobic culture plates can be placed in an anaerobic incubator, or a mini-anaerobic environment can be created in gas jars filled with specific mixtures of piped gases, or by using sachets containing ascorbic acid and activated carbon, which react on contact with air, absorbing oxygen and producing CO2.
For normally sterile tissues and fluids, an additional enrichment step is used wherein fluid or homogenised tissue is placed in enrichment broth incubated at 37°C, for 48 hours, after which 10μl of enrichment broth fluid is sub-cultured on to appropriate media (usually Colombia blood agar and anaerobic agar). Enrichment cultures are used in samples with low bacterial loads to increase a small number of organisms to detectable levels.
The plates are visually inspected to determine whether a pure or mixed growth exists (Figure 2) and the latter will require sub-culturing (purity plating). Individual colonies are taken from the plate, and streaked on to new plates and incubated again, to obtain a pure growth.
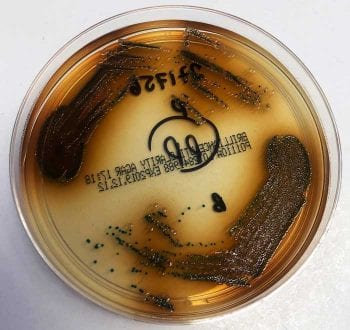
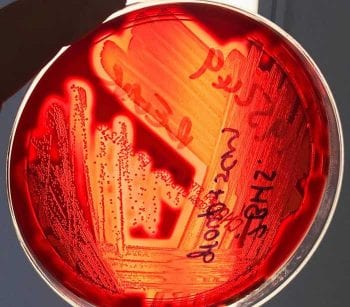
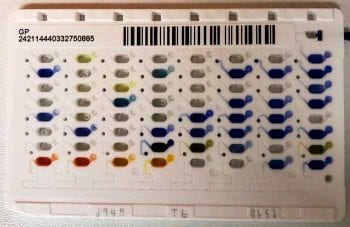
Bacteria are identified by assessing the appearance of the colonies, performing Gram stains and with a series of biochemical tests. Initially, a Gram stain and manual biochemical tests, such as oxidase and catalase tests, are performed. Gram-positive cocci can be staphylococci (which are catalase positive) or streptococci/enterococci (which are catalase negative). The pattern of haemolysis on the blood agar plates helps differentiate different types of streptococci and enterococci (Figure 3). These preliminary results, as well as the appearance of the colonies, will determine selection of subsequent choice of biochemical colorimetric test strips (Figure 4) or cards used in automated systems such as the Vitek analyser (Figure 5).
A suspension of bacteria is prepared to a standardised concentration using a turbidity meter and applied to test wells in colorimetric strips or loaded into the automated analyser. The strips consist of 10 to 50 wells, each containing different biochemical substrates. After adding the bacterial suspension, the strips are incubated before reading the colour change results. These are entered into a web-based database that uses algorithms to generate the identification. Automated systems use test cards with a greater variety of different biochemical tests (for example, Vitek cards have 64 testing wells), giving improved specificity. These tests measure various metabolic activities such as acidification, alkalinisation, enzyme hydrolysis and growth in the presence of inhibitory substances. During incubation, each test well is read every 15 minutes to either measure turbidity or analyse the coloured products of substrate metabolism. Again, the identification of the isolate is determined using algorithms.
In both methods, the level of confidence is reported and if this is low, further testing should be considered. The gold standard method of bacterial identification is matrix-assisted laser desorption/ionisation time of flight analysis. In these analysers, a suspension of bacteria is mixed with a matrix. A laser is directed at this matrix causing ionisation of bacterial molecules, which are desorbed (released from the matrix surface).
A potential difference is applied between the matrix source and a detector, causing ionised molecules to fly to the detector. Based on the time of flight, a characteristic spectrum called peptide mass fingerprint (PMF) is generated. The PMF of the unknown organism is compared with PMFs in a large database to produce the identification.
Gram-negative rods are divided into those that ferment glucose (Pasteurella and Enterobacterales, previously known as Enterobacteriaceae) and non-fermenters. Enterobacterales include Escherichia coli, Klebsiella, Proteus, Enterobacter, Citrobacter and Serratia. These organisms commonly cause urinary tract, wound, soft tissue, body cavity and ear infections.
Non-fermenters include Pseudomonas aeruginosa (a common cause of ear, eye and respiratory infections), Acinetobacter and Burkholderia (which can cause nosocomial infections). Non-fermenters have extensive intrinsic resistance to multiple antibiotics. Gram-negative cocci include Neisseria (which can cause oral infections) and Moraxella, which cause respiratory infections. Gram-negative coccobacilli include Bordetella and Haemophilus, which also cause respiratory infections.
Gram-positive cocci include staphylococci and streptococci, which cause infections in skin, ears, joints, urine, and are common causes of surgical site infections; and enterococci, which cause infections in many sites and have a wide range of intrinsic resistance. Gram-positive rods include Actinomyces (which often cause soft tissue abscesses or pyothorax) and Corynebacterium (which can cause ear infections).
Anaerobic bacteria can be isolated from various sites including the lung (due to aspiration pneumonia), pyothorax and abscesses – for example, tooth root abscesses, retrobulbar abscesses, bite wounds, soft tissue infections associated with plant foreign bodies, septic peritonitis, pyometra and infections in bone, liver, bile or the reproductive tract. Many infections involving obligate anaerobes also contain aerobes or facultative anaerobes.
The most commonly isolated obligate anaerobic bacteria are Bacteroides, Fusobacterium, Porphyromonas, Prevotella and Proprionibacterium species (Gram-negative rods), Peptostreptococcus species (Gram-positive cocci) and Clostridium species (Gram-positive rods). Periodontal infections are typically caused by anaerobes – especially Actinomyces odontolyticus.
Anaerobic infections are most often treated empirically because performing susceptibility testing on anaerobes is technically difficult and may require extended time before results are available, or may be unsuccessful due to the poor growth of the isolated anaerobes. Due to the largely uniform therapeutic treatment of anaerobic infections, mixed cultures are not usually separated and identified individually. However, this can sometimes be possible, depending on the nature of the organism in vitro. Anaerobes show predictable susceptibility to certain antibiotics including metronidazole, clindamycin and amoxicillin clavulanate, as well as pradofloxacin.

Antibiotic susceptibility testing (AST) is performed with disc diffusion (also known as Kirby-Bauer testing), or automated serial broth dilution tests that, in veterinary medicine, are usually performed with a Vitek analyser. A third method is the antimicrobial gradient method using e-tests.
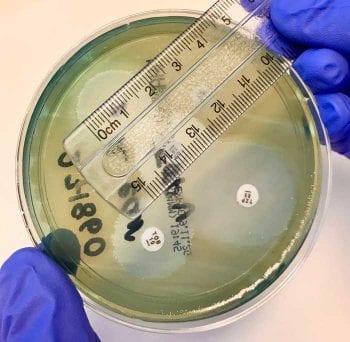
For all methods, a suspension of bacteria is prepared to a standardised concentration using a turbidity meter. In disc diffusion tests, the suspension is uniformly streaked on specified Mueller-Hinton agar plates and antibiotic discs containing a known concentration of antibiotic are applied at standardised distances from one another with no more than six discs on a 10cm plate. The plate is incubated for 16 to 20 hours, after which the zone diameters (diameter of the area of no bacterial growth around the disc) are measured using either a ruler or callipers (Figure 6).
The results are used to determine if the isolate is susceptible (sensitive), intermediate or resistant, based on the clinical breakpoint diameters provided by CLSI or EUCAST guidelines. Zone sizes differ for each antibiotic and also vary depending on the organism, so identification of an isolate prior to AST is essential.
For broth dilution testing, a known concentration of isolate suspension is incubated with doubling dilutions of antibiotics. The minimum concentration of antibiotic that inhibits bacterial growth (the minimal inhibitory concentration; MIC) is determined and again compared to published breakpoints. Automated analysers use small, disposable, plastic “microdilution” trays containing tiny wells and bacterial growth is measured by assessing the turbidity of the wells containing different concentrations of antibiotics. The higher the level of bacterial growth, the more turbid the suspension.
As an example, a Proteus species was isolated from canine urine. The zone size for enrofloxacin was 26mm, which is larger than the CLSI breakpoints shown in Table 1, indicating the organism is susceptible to enrofloxacin. Using broth microdilution, the MIC for enrofloxacin is 0.25μg/ml, which is lower than the breakpoint shown in Table 1, indicating the organism is susceptible.
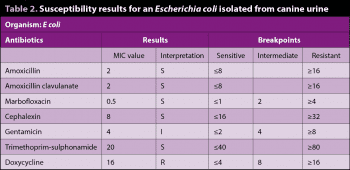
The MICs can be used to determine the antibiotic to which the organism is most susceptible (this is not possible with disc diffusion testing). Table 2 shows results for an E coli isolated from canine urine.
For those antibiotics with a susceptible result, the MIC of the isolate is compared with the breakpoint MIC, and the drug with the MIC at the highest number of doubling dilutions away from the breakpoint is likely to be the most effective (assuming it is concentrated at the target site).
In the example, amoxicillin and amoxicillin clavulanate both have an MIC of 2, which is 2 dilutions away from their breakpoints (8 ÷ 2 ÷ 2 = 2), while the MICs for marbofloxacin, cephalexin, and trimethoprim sulphonamide are only one dilution away from their breakpoints. Hence, in this case, amoxicillin would be the drug of choice, reserving amoxicillin clavulanate for treating infections producing beta lactamase. It is important to be aware that the drug with the lowest MIC is not necessarily the most suitable.
For both disc diffusion and broth dilution methods, breakpoints are set by EUCAST and CLSI. These guidelines take into account multiple factors, such as resistance mechanisms, pharmacokinetics, pharmacodynamics, clinical outcome and epidemiological cut-offs (ECOFFs). The latter are determined by assessing the wild type distribution for a particular drug-bug combination. The wild type population of a given organism is one that does not have acquired mutational resistance to the drug. The ECOFF is the upper limit of MIC for the wild type population.
A bacterium with a drug MIC greater than the ECOFF is likely to have an acquired form of resistance, whereas one with a drug MIC lower than or equal to the ECOFF is likely from the wild type distribution of the bacterium for a particular drug. The ECOFF is not the same as a susceptibility breakpoint and, as aforementioned, the breakpoint depends on other factors such as drug pharmacodynamics and dose, site of infection and so on. Breakpoints differ for different bug-drug combinations and are regularly updated, so it is important for the laboratory to ensure correct breakpoints are being used.
Intrinsic resistance refers to resistance resulting from the normal physiology or structure of the organism, and does not depend on the organism being exposed to the antibiotic. For example, enterobacterales and non-fermenting Gram-negative bacteria are intrinsically resistant to clindamycin, erythromycin and fusidic acid. Pseudomonas aeruginosa is also intrinsically resistant to amoxicillin-clavulanate acid, first to third-generation cephalosporins, tetracyclines, trimethoprim sulphonamide and chloramphenicol. Intrinsic resistance tables can be found on the EUCAST website (2019b).
Antibiotics for which intrinsic resistance is known are not tested, and a resistant result is issued. Susceptibility for some drugs is inferred from results of drugs in the same class – for example, for Streptococcus, benzylpenicillin susceptibility is used to predict susceptibility to all penicillins and cephalosporins.
For staphylococci species, two important resistance mechanisms exist: beta lactamase production and alteration of the drug binding site, penicillin-binding protein (PBP). Beta lactamase leads to resistance to ampicillin and amoxicillin, but susceptibility to amoxicillin clavulanate, cephalexin and higher generation cephalosporins.
Meticillin-resistant staphylococci (MRS) have altered PBP, which renders all beta lactam antibiotics (including carbapenems) resistant – the only exception being ceftaroline (which should not be used in animals). Beta lactamase production is evaluated with benzylpenicillin, while meticillin resistance is evaluated with oxacillin (for Staphylococcus pseudintermedius) or cefoxitin (for Staphylococcus aureus and coagulase negative staphylococci). MRS may be resistant only to beta lactams or may be resistant to drugs in other classes due to concurrent expression of other resistance genes.
Streptococci species generally have very low levels of resistance. Conversely, enterococci are intrinsically resistant to cephalosporins, clindamycin, erythromycin and sulphonamides, and may acquire resistance to penicillins and tetracyclines, with more frequent resistance to fluroquinolones.

Gram-positive bacteria may have inducible clindamycin resistance, which is assessed with a combination of clindamycin and erythromycin. In disc diffusion testing, the erythromycin disc is positioned adjacent to the clindamycin disc. A flattening of the zone of inhibition around the clindamycin disk on the same size as the erythromycin disk (producing a zone of inhibition shaped like the letter D) is considered a positive result. Where inducible resistance is detected, the clindamycin is reported as resistant and should not be used.
Beta lactamase resistance is problematic in Gram-negative bacteria, which are often resistant to amoxicillin and cephalexin. Some produce extended spectrum beta lactamases and ampC beta lactamases, rendering them resistant to penicillins and first to third-generation cephalosporins. These enzymes are encoded on plasmid genes, which spread between bacteria via conjugation (ampC enzymes can also be found on chromosomal genes).
Multiple antibiotic-resistant genes are often co-localised on the same plasmid, which means resistance to other antibiotics such as aminoglycosides, trimethoprim sulphonamides, tetracyclines and fluoroquinolones – and occasionally carbapenems can develop.
If more than one antibiotic has tested susceptible, several factors should be considered when selecting the most appropriate antibiotic. Antibiotics concentrated at the site of infection should be chosen – for example, drugs concentrated in urine (beta-lactams, fluoroquinolones or potentiated sulphonamides) should be used for urinary tract infections and the latter two used for prostatic infections since these cross the blood-prostate barrier.
For serious infection – especially sepsis – bactericidal drugs should be selected over bacteriostatic drugs. If MIC data is available, the drug with the MIC furthest away from the breakpoint is selected, as illustrated in Table 2. In the interests of antibiotic stewardship, the lowest generation effective drug in a given class should be selected (for example, cephalexin over cefovecin). The cascade should be considered.
The VMD issued a position statement (GOV.UK, 2014) stating it is justified, on a case-by-case basis, to prescribe an antibiotic under the cascade in the interests of minimising the development of resistance. This applies particularly where culture and sensitivity data indicate that a particular antibiotic active substance is effective against a bacterial pathogen.
Further guidance on prescribing antibiotics can be found via the BSAVA website (2019), which also has a link to the PROTECT ME poster summarising these guidelines.