13 Mar 2017
Michał Mól describes the diagnostic imaging modalities and medical undertakings regarding a case of a five-month-old puppy with hindlimb tissue damage.

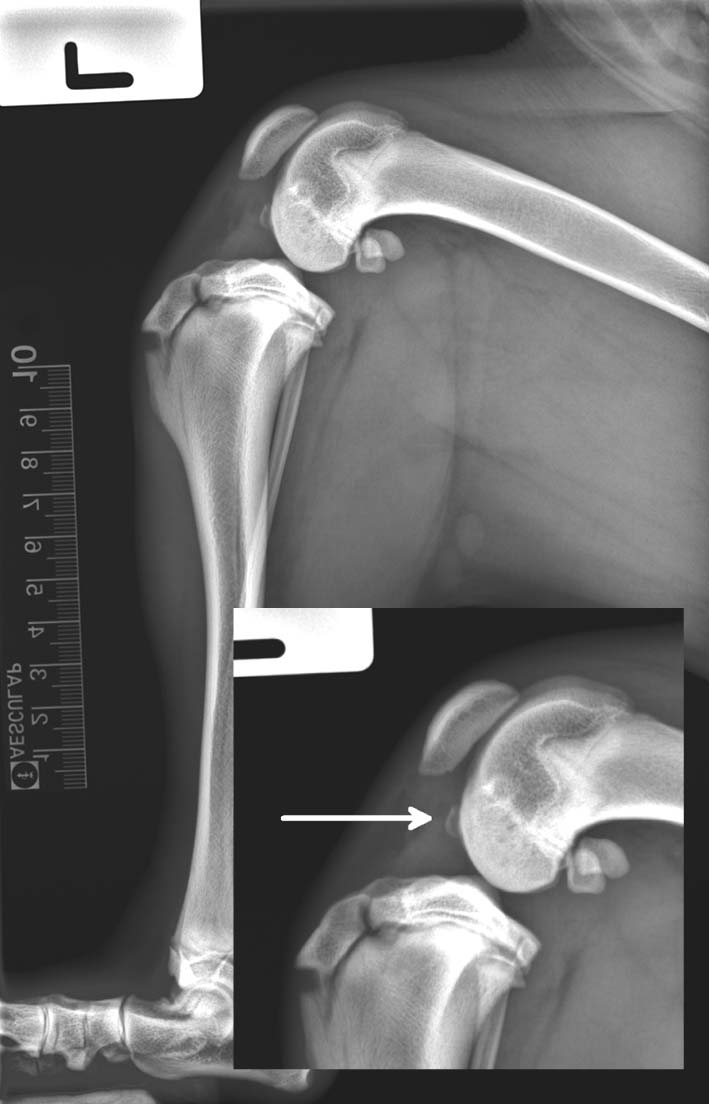
Figure 4. Mediolateral and caudocranial radiographic views of the left stifle – postoperative radiographs showed correct placement of the osteotomy and implants.
A five-month-old, male Labrador retriever weighing 20kg was referred with hindlimb weight-bearing lameness of two weeks’ duration. Joint effusion of the affected stifle was noted on the radiological examination. Arthrocentesis was performed prior to referral and the left stifle synovial fluid results were unremarkable. The dog had been treated with meloxicam daily.
A physical examination revealed external rotation of the left stifle, discomfort upon stifle palpation, moderate muscle atrophy and palpable joint effusion. Moderate cranial drawer movement with a soft end point was discovered during manipulation of the left stifle. A well-defined osseous fragment in the central aspect of the joint and a moderate amount of joint effusion were present on assessment of the radiographs. An MRI showed a bony fragment in the stifle joint attached to the cranial cruciate ligament with an associated concavity in the cranial aspect of the intercondylar region of the tibial plateau.
An arthrotomy was performed to inspect the cruciate ligaments and menisci. It confirmed the osseous fragment was caused by a partial cranial cruciate ligament avulsion. Closing tibial wedge osteotomy was performed. Good limb function was noted on the physical examination eight weeks postoperatively. Radiographs revealed complete bony union and implants were maintained in place.
The aetiology, diagnosis and treatment of cranial cruciate ligament disease are discussed, with an emphasis on methods and reliability of diagnostic imaging modalities used to investigate canine stifle conditions.
The stifle joint is anatomically complex and technically challenging to evaluate by the diagnostic modalities as it is stabilised by a compound arrangement of osseous, articular, fibrocartilaginous and ligamentous structures.
Mineralised opacities in the stifle most commonly result from trauma and are usually either avulsed:
Avulsed fragments might originate from:
Other conditions where intra-articular or peri-articular mineralisation in dogs might occur are:
Diverse tissue types, the small size of its structures and their superimposition limit successful diagnostic imaging, with a single modality in many conditions. The diagnostic imaging modality that addresses the properties of the tissues and pathology being examined will augment successful case outcome.
Various surgical methods have been described to treat cranial cruciate ligament (CrCL) insufficiency and restore joint stability, including extracapsular, intracapsular and tibial osteotomy techniques. MRI has recently been defined as the “gold standard” in human medicine because of the flexibility it affords the clinician when faced with imaging several different tissue types in a single structure.
A five-month-old, intact, male Labrador retriever was referred for weight-bearing left hind lameness of two weeks’ duration. No history of trauma was reported. The dog was initially treated with meloxicam 1.5mg/ml (0.1mg/kg once a day by mouth).
Radiographs taken prior to referral showed marked joint effusion. Arthrocentesis was performed at the primary care practice and the synovial fluid cytology from the affected joint was unremarkable. On reviewing the radiographs, an effusion in the left stifle and a bone fragment were present.
Observation of the gait revealed moderate unilateral left-sided pelvic limb grade 4/5 lameness (Innes et al, 2003) with toe touching and stifle external rotation. Moderate muscle atrophy of the affected limb was evident. The dog was normal weight, weighing 20kg. The dog was alert and responsive. The heart rate was 120 beats per minute with no audible murmur. No pulse deficits were detected. Respiratory rate was 36 breaths per minute and the dog was normothermic.
A physical examination revealed moderate discomfort on left stifle manipulation and palpable joint effusion was detected. A partial cranial drawer movement with a soft end point was discovered during manipulation of the left stifle. Tibial compression test (TCT) was negative. Neurological evaluation of the spine, trunk, pelvic limbs and tail applying cutaneous trunci and panniculus reflex, postural reactions and spinal reflexes was within normal limits.
The differential diagnoses of a mineralised opacity in the stifle (Panel 1) associated with joint effusion were documented and the dog was admitted for further investigation and treatment. Biochemistry and haematology were unremarkable.
A 20-gauge IV catheter was placed in the left cephalic vein and sedation was induced with medetomidine 1mg/ml (25μg/kg IV) and methadone 10mg/ml (0.2mg/kg IV). General anaesthesia was induced with propofol 10mg/ml (4mg/kg IV).
A 12mm cuffed endotracheal tube was placed and anaesthesia was maintained on 2% isoflurane in oxygen using a circle circuit and a 3L reservoir bag.
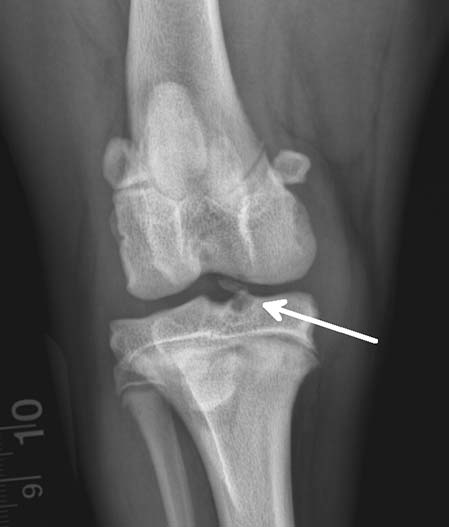
A mediolateral radiographic projection of the left stifle revealed a 6.3mm x 2.9mm osseous body in the cranial aspect of the joint in close proximity to the condyles and a moderate amount of synovial effusion (Figure 1). A caudocranial projection of the right stifle (Figure 2) showed the osseous body in the central aspect of the joint – between the femur intercondylar notch and tibial intercondylar eminence – suggesting the calcified body had originated from the tibia plateau insertion of the CrCL.
An additional diagnostic imaging – MRI studies – was performed using different sequences (Panel 2; Figure 3). The dog was positioned in right lateral recumbency and was scanned using an eight-channel wrist coil (receive only). This small coil was used to obtain the best quality images, by maximising signal-to-noise ratio. Sagittal proton density view with fat saturation (PDFS) demonstrated joint effusion, hyperintensity surrounding concavity in the cranial aspect of the intercondylar region of the tibial plateau; origin of CrCL. The cranial cruciate ligament appeared irregular with mixed signal intensity. An avulsed fragment was seen and attached to the cranial cruciate ligament – on coronal PDFS image – with an associated concavity at its insertion in the cranial aspect of the intercondylar region of the tibial plateau. The meniscus was normally defined and regular, and had uniform low density on all imaging sequences.
A provisional diagnosis of CrCL avulsion was made based on the history, physical examination, positive cranial drawer test findings, and radiographic and MRI features.
The closing tibial wedge osteotomy (CTWO) surgical technique of treatment of CrCL injury in a skeletally immature dog was chosen. The CTWO method can be performed in young dogs prior to closure of the proximal tibial physis, whereas tibial plateau-leveling osteotomy (TPLO) is not recommended for dogs younger than eight to nine months of age because of potential injury to the proximal tibial physis (Odders et al, 2004). The client was explained the goals of surgical stabilisation of a CrCL deficient stifle are to re-establish normal kinematics of the stifle and thereby mitigate the progression of OA.
Immediately following diagnostic imaging, the dog received meloxicam 5mg/ml (0.2mg/kg IV) and cefuroxime (20mg/kg IV). Administration of cefuroxime was repeated at 90-minute intervals during the surgical procedure on a prophylactic basis. Epidural analgesia was achieved with a combination of morphine sulphate 10mg/ml (0.1mg/kg) and bupivacaine hydrochloride 5mg/ml (0.5mg/kg) administered as a lumbosacral epidural injection. The left pelvic limb was clipped, from the coxofemoral joint to 2cm below the tarsus, and prepared for aseptic surgery using 4% chlorhexidine gluconate.
The dog was positioned in dorsolateral oblique recumbency with the affected limb lowermost and the contralateral limb extended cranially.
A skin incision was made medially from approximately the distal quarter of the femur to the proximal third of the tibia. A medial parapatellar arthrotomy was performed and inspection of the intra-articular structures confirmed the presence of an avulsed osseous fragment attached to the distal aspect of the CrCL. The rest of the CrCL was grossly abnormal with several small tears; the remnants were resected. Inspection of the menisci was unremarkable. A routine CTWO procedure was carried out using 3mm standard locking TPLO plate. The tibia plateau was changed from an angle of 25° pre-operatively to an angle of 5° postoperatively. The cranial margin of the sartorius muscle was sutured to the superficial fascia with 2-0 polydiaxanone with simple interrupted technique. SC tissues were re-apposed with a single continuous 2-0 poliglecaprone 25 and the skin was closed in a subdermal pattern using polyglytone 6211 suture. Postoperative radiographs showed correct placement of the osteotomy and the implants. Cryotherapy was applied prior to placement of a 24-hour Robert Jones bandage.
Routine suture removal was performed 12 days postoperatively and grade 2/5 lameness was documented following gait assessment. Mediolateral and caudocranial radiographic views of the left stifle were obtained six weeks postoperatively (Figure 4) using the same TPLO protocol as described previously. No residual lameness was reported by the owner, or noted on physical examination. The patient had full range of motion at the affected stifle joint. The radiographs revealed uniform fusion of the previous bone defect; implants were maintained in place.
The canine stifle represents a diagnostic imaging challenge because of its complex composition. Diagnosis in dogs with the CrCL disease is usually based on physical examination with demonstration of laxity of the joint by cranial drawer or tibial compression tests in combination with diagnostic imaging. Radiography is widely available and is still the most commonly used diagnostic imaging aid in the detection of CrCL disease in dogs. Radiographic signs can include increased synovial fluid volume, thickening of the synovial lining, cranial displacement of the tibia in the mediolateral view with tarsal flexion applied (Cazieux-positive sign), compression of the infrapatellar fat pad and, in chronic cases, osteoarthritic changes. These changes might include mineralisation of soft tissues, intra-articular mineralisation or joint malformation and decreased or increased subchondral
bone opacity.
CrCL injury can occur at any age and has the greatest incidence in large and giant breed dogs (Duval et al,1999). Cranial cruciate avulsions are uncommon injuries, being reported most often in young dogs. CrCL rupture in puppies is usually associated with traumatic injury and avulsion of the ligament from its sites of attachment. The cranial cruciate ligament originates on the medial surface of the lateral femoral condyle and extends in a cranio-medio-distal direction to insert on the cranial aspect of the intercondylar region of the tibia plateau.
In skeletally immature animals, the bone is relatively weak compared to the ligaments/tendons and avulsion fractures may occur, with small fractures arising at the origin or insertion of the ligaments/tendons. Breaking strength of the cranial cruciate ligament – the force necessary to cause acute ligamentous rupture – has been suggested to be approximately equal to four times the bodyweight of the dog (Williams et al, 1997).
The radiographic changes support the CrCL avulsion diagnosis, but MRI images were much more sensitive and, therefore, able to confirm the fragment was associated with the cranial cruciate ligament. For most diseases of the stifle, radiography may be sufficient to make a diagnosis when clinical signs and physical examination findings are included in the evaluation; however, if radiographic assessment is inconclusive, more advanced imaging is necessary.
Tissues do not absorb x-rays uniformly; radiographic images have regions of white, black, and shades of grey. Bone absorbs more x-rays than soft tissue, resulting in images that are radiopaque, whereas soft tissue appears as shades of grey. These properties of energy absorption confirmed the presence of the osseous body and increased synovial fluid volume.
1. Localiser – transverse standard gradient echo (GE) localiser. It runs automatically at centre of coil.
2. Localiser – transverse, coronal, sagittal, standard GE localiser.
3. Transverse T1-weighted images:
4. Coronal PDFS:
5. Sagittal PDFS:
6. Coronal short T1 inversion recovery:
In addition to detecting the pathology, preoperative radiographs were needed to plan the surgery. The mediolateral (TPLO) radiographs were used to plan the position and angle for the osteotomy and the position of the implants relative to the stifle joint. The caudocranial view helped identify the palpable landmarks that can assist the surgeon in avoiding intra-articular placement of screws and pins. Standard radiographic projections are also used to determine if further diagnostic tests are needed (Figure 5).
Joint fluid analysis and cytology, immune profile and infectious titres are used in conjunction with radiography to differentiate possible causes of joint inflammation.
The soft tissue not evident radiographically – the straight patella ligament, thickening of the joint capsule or cartilage defects – can be detected by ultrasonography. However, ultrasonography has a low sensitivity for detection of cranial cruciate rupture (Arnault et al, 2009).
CT of the stifle has been used; it is a minimally invasive and repeatable technique that does not require general anaesthesia. Although CT scanners have been used frequently in human medicine to assess intra-articular structures and have the benefit of providing better osseous visualisation and shorter scan times when compared with MRI, most stifle imaging studies in veterinary medicine are centred on the assessment of intra-articular ligamentous abnormalities making MRI a more suitable modality (Marino and Loughin, 2010).
MRI provides superior soft tissue contrast to CT in the absence of ionising radiation. MRI is becoming an increasingly popular method of evaluating many types of musculoskeletal disease. MRI has been defined as the “gold standard” in human medicine because of the flexibility it affords the clinician when faced with imaging several different tissue types in a single structure. Thermal imaging offers the advantage of objectively viewing physiological changes within the anatomical region of interest before the onset of structural change.
The major advantages of MRI are its excellent image resolution, superior soft tissue contrast, acquisition of images in any plane and use of a magnetic field rather than ionising radiation. Because protons in different tissues realign at different rates, the radiofrequency (RF) signal received by the RF coil can be filtered to accentuate different tissue characteristics using specific “sequences”. With the introduction of region-specific surface coils and stronger magnets, improved signal-to-noise ratio, enhanced spatial resolution and abbreviated scan times, MRI is now commonly used in people to assess internal derangements of the knee, wrist, hip, hand and shoulder.
MRI evaluation of the internal architecture of the stifle joint affords many advantages over arthroscopy or arthrotomy and is the primary imaging modality when assessing for cruciate, meniscal and articular pathology in people. Cranial and caudal cruciate ligaments are clearly depicted in our case; their integrity was assessed based on changes in shape, contour, size and signal intensity. The presence of cruciate ligament damage and meniscal tears can be accurately assessed using the MRI images; however, in the case of meniscal evaluation, occasional misdiagnosis occurs (Galindo-Zamora et al, 2013).
In conclusion, while radiographic images are helpful to evaluate bone structures and are needed to plan the surgery, soft tissue arrangement of the stifle joint – including joint capsule and synovium, ligaments, menisci and cartilage – all appear as a uniform opacity on the radiographs that contour with the joint fluid.
MRI offers great possibilities in helping overcome clinical inconclusiveness. The key to successful management of the diagnostic options available is to have a thorough understanding of the anatomy and tissue properties of the region being imaged and to recognise the strengths and weaknesses of the modality being selected. Ultimately, a multimodality approach will likely provide a complete assessment of complex structures by combining the strengths of each modality to visualise the tissue characteristics of the structure being imaged.
The author would like to thank Heather Jones for her help with this article.