20 Aug 2018
Diana Ferreira describes approaches to the control of flare factors and chronic management of this lifelong skin disease in dogs.

Figure 1. A Jack Russell terrier with chronic atopic dermatitis showing self-induced alopecia in the flank and paws, and periocular hyperpigmentation.
Canine atopic dermatitis (CAD) is the most common allergic dermatitis in dogs. The pathogenesis is complex and multifactorial, with genetic and environmental factors involved in the determination of susceptibility to developing the clinical disease. CAD is a chronic, lifelong disease. Although a cure is rarely obtained, it can be successfully managed through a combination of therapeutical actions. However, multiple pathophysiological mechanisms are involved in the development of clinical signs, which means a treatment effective for one patient may not be for another.
The traditional treatment approach is based on slowing down the inflammatory process associated with the disease – and, therefore, reducing the inflammation and pruritus, which can result in self-trauma and, consequently, the rapid development of secondary infections that further aggravate the clinical signs.
It is also important to identify and control the flare factors that act as triggers of acute disease, such as fleas, foods, secondary infections and environmental allergens. Long-term management of the disease should result in a reduction of the frequency of new flares and bring stability to the disease. Ultimately, the treatment approach to CAD is always multimodal and should be individually tailored for each patient.
Canine atopic dermatitis (CAD) is the most common allergic dermatitis in dogs. The pathogenesis is complex and multifactorial, with genetic and environmental factors involved in the determination of susceptibility to developing the clinical disease.
Sensitisation to environmental allergens leads to cutaneous inflammation. Several factors contribute to the development of sensitivities – one of the most important being dysfunction of the epidermal barrier.
The diagnosis of CAD in dogs is based on the presence of characteristic clinical features and the exclusion of diseases that result in a similar clinical presentation. The diagnosis, therefore, is clinical. Allergy tests are, therefore, not used as diagnostic tools and contribute only for making targeted therapeutic decisions.
CAD is a chronic, lifelong disease. Although a cure is rarely obtained, it can be successfully managed through a combination of therapeutical actions individually tailored for each patient.
Clinical signs in atopic dogs usually develop between six months and three years old.
The most important clinical feature is pruritus, which can be associated with lesions such as erythematous macules and papules, and with a characteristic distribution affecting the face, flexor aspects of the elbows, paws, ventral abdomen, perineum and ear canals. With chronicity, the ongoing pruritus and inflammation can result in the development of lesions such as excoriations, self-induced alopecia, lichenification and hyperpigmentation (Figure 1).
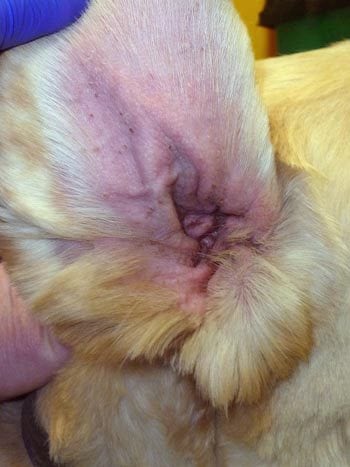
Secondary Malassezia dermatitis (Figure 2), bacterial infections and otitis externa (Figure 3) occur commonly in dogs with CAD.
The traditional treatment approach is based on slowing down the inflammatory process associated with the disease, thereby blocking the allergic sensitisation.
Throughout the past decade, the importance of restoring the skin barrier function in CAD has also been recognised. In atopic dogs, cutaneous barrier dysfunction facilitates the penetration of allergens and increases the risk of allergic sensitisation.
Most atopic patients will concurrently suffer from conditions that act as flare factors, ranging from concomitant allergies to secondary bacterial and yeast infections, which, in addition to contributing to inflammation, aggravate pruritus.
For all these reasons, the treatment approach to CAD is always multimodal, where the control of flare factors becomes as important as controlling the inflammation and pruritus. CAD is chronic, and recurrent and therapeutic management should not only aim at controlling the acute flares, but also the long-term management of the disease. The ultimate goal should focus on reducing the frequency of new flares.
To manage acute flares, it is important to first identify the factors that trigger them. These are fleas, foods, secondary infections and environmental allergens.
The second step is to initiate a treatment that immediately relieves symptoms, and reduces inflammation and pruritus, which can result in self-trauma and, consequently, the rapid development of secondary infections that aggravate the clinical signs more.
For immediate relief, short-acting glucocorticoids are one of the treatments of choice. Oral short-acting glucocorticoids – prednisone or prednisolone being the preferred options – are used at a dose between 0.5mg/kg to 1mg/kg. Oral methylprednisolone between 0.4mg/kg to 0.8mg/kg can also be used.
Glucocorticoids have the advantage of having a fairly fast action and are not expensive; however, the side effects associated with their use represent an important disadvantage. Polyuria, polydipsia and polyphagia are dose-dependent; however, with chronic use, obesity, muscle and skin atrophy, behavioural changes, predisposition for secondary infections, demodicosis and iatrogenic hyperadrenocorticism can impede their use.
These medications can initially be administered once daily, or the dose divided into twice daily for the first week, followed by a gradual reduction of the dose and frequency.
Oclacitinib is an effective and fast-acting treatment representing a good alternative to glucocorticoids. The recommended dose is 0.4mg/kg to 0.6mg/kg twice daily for the first two weeks of treatment, followed by a once-a-day regimen.
The occurrence of adverse effects associated with its administration is low, which represents a considerable advantage over glucocorticoids. Adverse effects are seen in 2% of patients and mainly include anorexia, vomiting and diarrhoea. Many patients experience an aggravation of symptoms when the regimen is altered to once daily; however, a gradual return to an acceptable level of pruritus over time is expected.
Lokivetmab represents another therapeutic alternative to be considered. Lokivetmab is an anti-interleukin (IL)-31 monoclonal antibody. IL-31 is a recently identified inflammatory mediator that seems to have a critical role in the development of pruritus in the atopic dog. This medication is given SC and aims to block the action of circulating IL-31.
This therapy is effective in approximately 80% of patients and has a duration of action of one month. It is also a safe treatment, being associated with a very low rate of side effects. Rare cases are described of hypersensitivity reactions to lokivetmab, including anaphylaxis, facial oedema and urticarial.
Topical therapies should also be considered for the management of acute flares of CAD. Topical glucocorticoids can be used for the treatment of focal inflammatory lesions. Its long-term daily use can, however, result in skin atrophy.
Ciclosporin, at a dose of 5mg/kg/day, is to be considered in the chronic management of CAD. Compared with glucocorticoids, ciclosporin is equally effective and has a lower frequency of adverse effects.
However, its maximum action takes up to three to four weeks to occur, requiring, in some cases, the concurrent use of a faster-action drug, while ciclosporin effectiveness builds up. Glucocorticoids can be used in this initiation phase, along with ciclosporin. Oclacitinib, given in the first three weeks of treatment with ciclosporin, also seems to be safe, according to a study (Panteri et al, 2016).
Because ciclosporin has immunosuppressive properties, its long-term use may increase susceptibility to infections. It is, nevertheless, considered a safe drug for chronic use in CAD. Gastrointestinal signs – primarily vomiting and diarrhoea – are the most often related events, accounting for about 50% of adverse effects. Most of these adverse effects occur with daily dosing and tend to resolve with dose reduction or temporary discontinuation of the drug.
If maximal efficacy is observed after an initial treatment period of four to six weeks, the administration regimen can be altered. The dosage can be reduced by 25% or the frequency of administration can be decreased to alternating days. This dosage or frequency decrease can be repeated gradually every four to six weeks until the minimum effective dosage is found.
Oclacitinib can also be considered for the chronic management of CAD. A study (Cosgrove et al, 2015) assessed the long-term safety, efficacy and quality of life of 247 dogs with allergic skin disease treated with oclacitinib. The percentage of dogs showing a 50% or more reduction of pruritus and dermatitis was 63.9% and 66.4%, respectively.
Owners saw a positive impact on quality of life in more than 91% of all dogs, and haematology and serum chemistry means remained within the normal reference ranges, indicating oclacitinib is safe and efficacious for long-term use and improves the quality of life for dogs.
Systemic glucocorticoids can be considered as a long-term treatment when alternative interventions are not effective or too expensive. Short-acting oral glucocorticoids (prednisone, prednisolone and methylprednisolone) can be used with reasonable safety.
A typical regimen involves tapering the last daily dose to alternate days, followed by gradual tapering until the lowest effective dose is established. Dogs in this treatment protocol should be monitored with physical examination and urine culture at least every 12 months.
Lokivetmab is a fairly recent product. Safety and efficacy studies were carried out for a maximum of six months; therefore, no long-term information is known regarding its use.
It can, however, be considered for use in the long-term management of pruritus in dogs with CAD – either alone, or in conjunction with other treatment options when they are not completely effective, or when their use is reduced due to the occurrence of side effects.
Allergen-specific immunotherapy is considered the most appropriate therapeutic option for the chronic management of CAD. However, indications are restricted to young animals with clinical signs present for more than six months a year. This is the only therapeutic intervention that can alter the course of the disease. When effective, it leads to a decrease in the long-term need for immunomodulatory drugs.
It can be administered SC or applied in the mouth (sublingual) as a spray. Efficacy seems to occur sooner in the latter option (in the first six months of treatment) as opposed to the SC route, which can take up to a year to result in an improvement of clinical signs. The majority of patients will still need a concomitant symptomatic treatment (glucocorticoids, oclacitinib, lokivetmab and ciclosporin) in the first few months of treatment, while the effects of this therapeutic option are not observed.
Type one antihistamines are inexpensive and have a relatively good safety profile, but their efficacy in CAD is somewhat limited, possibly due to the fact histamine is not a major mediator for cutaneous inflammation and pruritus in this disease, and because antihistamines cannot exert their action once histamine has already bound to its receptors.
For these reasons, antihistamines are not usually recommended for treating acute disease, but may be used as part of a combination therapy approach for long-term management of CAD in an attempt to reduce the dose of other drugs, such as glucocorticoids. Antihistamines with existing pharmacological data in dogs include hydroxyzine (2mg/kg twice daily orally) and its active metabolite, cetirizine (1mg/kg once daily orally).
One strategy for combination therapy is to establish the minimum dosage of glucocorticoids that controls the clinical signs by sequential dose reduction, then reduce this dose by 50% and add an antihistamine.
Topical glucocorticoids can also be considered in the chronic management of CAD. A study in atopic dogs determined the use of a hydrocortisone aceponate spray in dogs in remission – applied twice weekly in areas predisposed to the development of lesions – decreased the time of relapse by four times (Lourenco-Martins et al, 2012). Although the occurrence of local adverse effects is unlikely with this frequency of application, it is advisable to monitor the development of lesions.
Interventions that aim to improve the barrier function of the epidermis should be considered in chronic management of the atopic dog. Skin barrier dysfunction occurs due to alterations in the cutaneous lipid metabolism, due to a reduced or altered synthesis of ceramides in the skin, as well as some essential proteins, such as filaggrin. These epidermal defects result in a drier skin that can contribute to, or aggravate, the pruritus and potentiate the increased penetration of allergens – leading to further sensitisation.
Baths with moisturising products that contain lipids have been shown to have a positive impact on lesions and pruritus in allergic dogs.
Oral supplementation with omega-3 and omega-6 fatty acids – both incorporated into the diet and administered as specific dietary supplements – are also a common therapeutic practice in dogs with CAD. The goal of this intervention is to decrease the production of pro-inflammatory eicosanoids – therefore inhibiting the activation of inflammatory cells and the production of cytokines – and restore lipid metabolism, with the final objective of normalising the stratum corneum.
Its effectiveness is, however, very slow and is why it is an intervention to be considered more for the chronic management of CAD.
The management of CAD should always be multimodal and tailored to each individual case. Although no cure exists for the disease, many advances have been made in recent years in respect to therapeutic options. Options should prioritise interventions that are effective as well as be associated with fewer side effects for the patient.