12 Feb 2018
Jenny Helm looks at an increase in reported cases of this disease in dogs, and describes how to diagnose, treat and prevent it.

Figure 1. Some outdoor pursuits also put humans at risk of Ixodes ricinus. Image © Schmutzler-Schaub / Adobe Stock
Alongside an increased understanding of Lyme disease and an expansion in the geographical distribution of infected ticks, public awareness of this disease has been raised through a number of high-profile celebrities talking about their diagnoses, including rugby star Matt Dawson; singers Shania Twain, Kelly Osbourne and Avril Lavigne; and actors Mark Ruffalo, Alec Baldwin and Kirstie Alley.
It is, therefore, timely to update the veterinary community about Lyme disease – including its clinical signs, diagnosis, and treatment – to ensure, as a profession, we can act to provide the correct advice to concerned pet owners, and improve our own understanding of a disease relevant to both human and animal health.
Lyme disease was first described in Old Lyme, Connecticut in 1976 – hence the name. However, the disease itself is much older, with human patients recognised in Germany from the late 1800s.
Lyme disease is caused by the motile, corkscrew-shaped spirochete bacteria Borrelia burgdorferi sensu lato (primarily B burgdorferi sensu stricto, B afzelii and B garini) and the bacteria is transmitted by ticks in the UK, mainly Ixodes ricinus (Panel 1).
Overall, 52 species of Borrelia bacteria exist, including 21 in the Lyme borreliosis group (B burgdorferi sensu lato). Within the group, the species B burgdorferi sensu stricto is the main pathogenic species in North America and Europe, of which at least 30 differing strains/subtypes exists based on genotyping.
Lyme disease is considered zoonotic, as ticks can feed on both animals and humans, and, as such, both can be affected by the disease. However, the disease mostly circulates in wild animal reservoirs and only rarely affects people.
Pets infected with Lyme bacteria do not usually pose a direct risk to their owners because of the tick’s long duration of life cycle spent off the host, although they may indicate an increased overall family risk due to shared activities or behaviours. Additionally, dogs may carry infected, unfed ticks into a household, which may, subsequently, attach to humans. It is known dog owners appear to be at greater risk of Lyme disease compared to non-dog owners (Smith et al, 2012).
Reported cases of Lyme disease in England and Wales have increased from 0.38 per 100,000 in 2000 to 1.59 per 100,000 in 2009 (Public Health England, 2013). In areas thought to be “high risk”, such as Scotland, the numbers of human cases may be even higher, with a prevalence of 1.25 to 16.5 cases per 100,000 in some studies (Davidson et al, 1999; Ho-Yen et al, 1999).
Factors underpinning this increase may include raised awareness, increased surveillance schemes and better reporting systems. Activities that may be more widely practised, with the increasing popularity of fitness and well-being, such as some outdoor pursuits, also put humans at risk of acquiring disease. Plus, evidence exists the primary vector – I ricinus – is becoming more abundant and more widely distributed (Figure 1).
Reasons for this expansion of ticks include:

Surveys have found 2.3 per cent of ticks retrieved from pet dogs in the UK were infected with B burgdorferi sensu lato (Smith et al, 2012).
In Europe, Lyme disease is most prevalent in central and eastern areas, although disease is certainly present in the UK. Information is not yet reliable about the “high risk” areas in the UK. It is dependent on the environmental risk (the density of infected ticks) and how people/pets interact with the environment – with awareness of ticks and tick-borne disease also playing a role.
Some texts state areas in the UK at higher risk may be the Scottish Highlands (Figure 2), Ireland, Wales, the Lake District, North Yorkshire Moors and the south-east of England (especially the New Forest, South Downs and Thetford Forest), which is based on the type of environment found in these locations, but other sources disagree.
Dogs of all breeds and ages are at risk, although young, large breed dogs with active and outdoor lifestyles may be at increased risk. Additionally, Bernese mountain dogs are more often seropositive, compared to other breeds in central Europe, although this seropositivity is rarely associated with illness (Gerber et al, 2007).
Seropositivity for B burgdorferi sensu lato is possible in cats, although associated clinical disease has not yet been reported.
In contrast to humans, dogs do not typically develop a skin rash (erythema migrans) at the site of an infected tick bite. In fact, only about 10 per cent of infected dogs show overt clinical signs at the time of infection.
Instead, if clinical signs occur, they appear two to five months after the tick bite and include pyrexia, lethargy, inappetence, thrombocytopenia, mild lymphadenomegaly and lameness (secondary to Lyme arthritis). Ocular, neurological and cardiac clinical signs, as seen in human infections, are uncommon in dogs.
Lyme nephritis is a type of membranoproliferative glomerulonephritis (thought to be secondary to immune-mediated mechanisms), which has been identified in dogs – particularly golden and Labrador retrievers, and possibly Bernese mountain dogs and Shetland sheep dogs – and very rarely in humans. Lyme nephritis tends to affect younger dogs (often less than five years old) compared to other types of glomerular disease, such as those caused by coinfections, neoplasia, genetic, toxic or other conditions. Clinical signs are secondary to glomerular protein loss and renal failure. They include anorexia, vomiting, lethargy, dehydration, polyuria, polydipsia and weight loss.
Lyme nephritis is still not fully understood. The disease has not yet been recreated under experimental conditions and, as such, Borrelia, as a causal agent, does not completely fulfil Koch’s postulates. Although subendothelial deposits of IgM, IgG and C3 antibody deposits have been found in the glomeruli of affected dogs, Borrelia DNA and antigen have not consistently been identified.
Lyme arthritis is most commonly a non-erosive inflammatory arthropathy that typically manifests as shifting lameness. The lameness may occur in the limb closest to the initial tick bite, although this is hard to confirm in dogs, given the lack of a skin rash, their haired skin and the delay in onset of clinical signs. Such episodes of lameness may last a few days and wax and wane over time.
It should be borne in mind many seropositive dogs and cats show no clinical signs of illness – either those that have been experimentally infected or those infected naturally in the field.
Other organisms can be transmitted by Ixodes ticks and can also be associated with similar clinical signs to borreliosis. As such, Anaplasma phagocytophilum, some Ehrlichia species and, potentially, some Bartonella species can be important differential diagnosis, or, if they exist as coinfections, may increase morbidity.
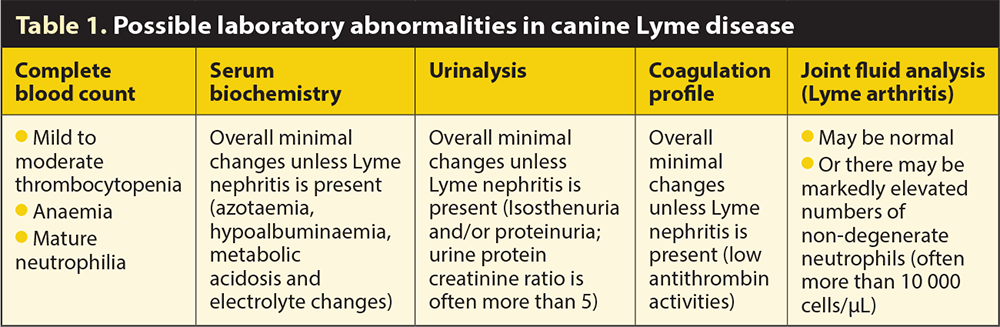
Routine laboratory abnormalities can occur, although they are certainly not pathognomonic for Lyme disease. These are shown in Table 1.
Findings on diagnostic imaging are non-specific. In dogs with Lyme arthritis, the only changes on plain radiography tend to be increased periarticular soft tissue opacity. Thoracic radiography is often entirely normal, although pleural effusion secondary to hypoalbuminaemia in Lyme nephritis is very rarely described.
Abdominal ultrasonography is usually normal unless Lyme nephritis exists, where they may be thickened and hyperechogenic renal cortices, decreased corticomedullary definition and, possibly, peritoneal effusion.
The complete genome of B burgdorferi has been sequenced and the differences in the outer surface lipoproteins at different life stages have been recognised, which have been exploited in diagnostic tests and in the production of vaccines. Important outer surface proteins (Osp) include OspA, OspC and variable lipoprotein surface-exposed (VIsE).
Over winter, late autumn and early spring, the bacteria are dormant in the midgut of nymphal ticks – at this time expressing OspA. Later, when the nymph ingests mammalian blood, the bacteria downregulates OspA expression and upregulates OspC, which, by binding to a salivary protein within the tick, helps Borrelia evade the host immune system, and in binding to mammalian plasminogen helps the organism distribute throughout the final host. VIsE is also involved in aiding evasion of the host’s immune response.
Confirming a diagnosis of Lyme disease can be challenging. In dogs, serology is a recommended first step to evaluate exposure. Because clinical signs usually occur months after exposure, false-negative serology results are incredibly unlikely. The difficulty lies in identifying dogs that have an active infection (and clinical signs attributable to this) and those that simply have previous exposure or infection with a non-pathogenic variant of the B burgdorferi sensu lato species. Dogs with previous exposure may have another reason for their clinical signs and, as such, further diagnostics may be required.
In making a diagnosis (Panel 2) several serological assays are available, the most widely used is based on a C6 ELISA, which detects antibodies against the C6 protein. This test is often combined as a rapid in-house assay to look for Ehrlichia canis/Ehrlichia ewingii antibody, Anaplasma species antibody and Dirofilaria immitis antigen. Although a positive result may need to be followed up with a quantitative antibody test, not enough evidence exists to suggest these titres are completely correlated to disease versus exposure.
Bacterial culture is not usually beneficial as it has low sensitivity. It is a very slow process, taking several weeks, and requires appropriate tissue – often a skin biopsy close to the tick bite, which may not be identifiable at the time of presentation. Additionally, isolation does not imply Borrelia is the causal agent of clinical signs. PCR performed on appropriate tissue – such as a skin biopsy or synovial tissue/fluid – to look for B burgdorferi DNA is rapid, but insensitive, because it is imperative the sample tissue actually contains the bacteria. As such this is an area that requires further study. PCR on blood is not usually performed due to its low sensitivity.
Treatment is based on appropriate antibiosis and management of clinical signs (providing analgesia and so on). Although the best antibiotic, dose or duration of treatment is not proven, most sources recommend doxycycline – due to its known efficacy against Borrelia and coinfectious organisms, and its anti-arthritic and anti-inflammatory properties – at 10mg/kg per os every 24 hours or 5mg/kg per os every 12 hours over 4 weeks. Some clinicians recommend the use of 4 weeks of amoxicillin or cefovecin (2 injections 14 days apart) in dogs sensitive to doxycycline, or in young, growing dogs.
If the clinical signs are attributable to Lyme disease the response to appropriate treatment (as above) should be rapid (within days). However, relapse is possible and may be due to coinfection or reinfection.
For Lyme arthritis, analgesia should be provided; opioid medications and/or gabapentin may be preferable to non-steroidal drugs given corticosteroids may be needed in some dogs that are non-responsive to antibiosis, due to an underlying immune-mediated aetiology of their polyarthritis.
Management of Lyme nephritis requires supportive care, as well as appropriate antibiotics. Such care could include an angiotensin-converting enzyme or aldosterone inhibitors, anti-hypertension medications, anti-coagulants, omega-3 free fatty acids, protein and phosphorus-restricted diets (further guidance on the management of glomerular disease is available elsewhere).
Anti-emetics, gastroprotectants, IV fluids and phosphate binders may also be indicated. Immunosuppressant therapy is usually reserved for dogs that have had renal biopsies and proven active immune-mediated glomerular disease. However, if dogs with protein-losing nephropathy are deteriorating despite appropriate care, advice should be sought from an expert, as immunosuppressants may be indicated in such cases.

Safe tick removal is essential; if a tick is unduly stressed or injured during removal, transmission of disease from the tick to the host animal is much more likely. Both the body and the small head and mouthparts, which are often buried in the host’s epidermis, must be removed in their entirety.
To avoid bursting or causing stress to the tick, older techniques, such as applying petroleum jelly, alcohol or burning, should be avoided. In ideal circumstances, a specific tick removal device should be used (Figure 3).
Although transmission times of borreliosis can be relatively long – up to 60 hours in one study (Piesman and Dolan, 2002) and typically 36 to 48 hours – reports suggest this may not always be the case (Kidd and Breitschwerdt, 2003; Nicholson et al, 2010) with other tick-borne pathogens.
The author would recommend, where feasible, ticks are removed as described previously as soon as they are observed and, if possible, within 24 hours. To prevent any attachment at all, owners should be encouraged to use veterinary-recommended tick repellent products for their pets.
When it comes to preventing tick infestation, many products are available and, more often than not, are tailored to treat many different ectoparasites and endoparasites. They fit well with modern society’s desire for convenience and time-saving innovations, but they do not always promote an all-round approach to strategic parasite control. Overall, it is important to recommend a product with proven efficacy and safety.
Tick prevention also includes careful grooming and monitoring. Owners should be encouraged to do this on a regular basis, especially during March to November when tick numbers increase; although, as ticks can be active even at low temperatures (above 4.5°C), year-round protection is advisable. Ticks can also attach to humans, so pet owners should carefully check themselves.
Vaccinating dogs against Lyme disease in the UK is not routine, but in many parts of the world where the disease is endemic (given that compliance to tick prevention products is never 100 per cent) many vets recommend it. Lyme vaccines stimulate the host’s immune response against bacterial surface proteins normally expressed when it resides within a tick. The hope is canine antibodies are ingested if an infected tick bites a susceptible host and the bacteria is destroyed within the tick before it can infect the host.
Several vaccines are available, but none give complete protection and some may have been associated with stimulating host autoimmune disease. Additionally, little is known about the benefits of vaccinating dogs already exposed to Borrelia and it is unknown if vaccination prevents some of the severe consequences of infection, such as Lyme nephritis.
Notably, the summary from the WSAVA‘s vaccination guidelines (Day et al, 2016) is that vaccination for Lyme disease (B burgdorferi) is non-core and should only be given to dogs with a known risk of exposure, living in or visiting areas where the risk of tick exposure or disease is high.
It is thought more than 90 per cent of dogs infected with B burgdorferi do not show clinical signs. In those that do show signs of disease, most recover rapidly with appropriate treatment. Conversely, dogs with Lyme nephritis typically have a guarded to poor prognosis.
Lyme disease poses a risk to both animals and humans in the UK. It is more important than ever the veterinary profession is aware of the risks and understands the disease to enable us to recognise it, treat it appropriately and prevent it wherever possible.