18 Nov 2019
James McMurrough discusses the approach to this issue – including bronchoalveolar lavage, when to perform it, what to test and how to interpret results.

Signs referable to lower respiratory tract disease are a common reason for patients presenting to the veterinary clinic.
The most common of these is a cough – although dyspnoea, syncope, collapse, hyporexia, lethargy and weakness are seen in some patients.
Lower respiratory tract disease can vary in severity – with some patients presenting with chronic, slowly progressive clinical signs, while others may present acutely with significant respiratory compromise requiring emergency therapy.
Patients with significant respiratory compromise are unable to undergo extensive diagnostic evaluation, meaning a tentative diagnosis and treatment plan is based on signalment, history, physical examination findings and response to trial therapy.
Patients with chronic lower respiratory signs often require extensive diagnostics to reach a definitive diagnosis.
It is important to be aware many causes of lower respiratory disease are not curable, and that reductions in cough frequency and severity may be the best that can be achieved with therapy (for example, chronic bronchitis).
Inflammatory:
Degenerative (or secondary lower airway diseases):
Neoplastic:
Traumatic:
Allergic:
Cardiovascular:
Parasitic:
The causes of canine lower respiratory tract disease are extensive, but include primary lower airway diseases, secondary lower airway diseases and primary pulmonary parenchymal diseases (Panel 1).
Some patients may have primary cardiovascular disease, which can mimic lower respiratory tract disease – for example, left mainstem bronchus compression due to significant left atrial cardiomegaly in patients with advanced mitral valve disease (Figure 1). Other patients may even have a combination of primary cardiovascular disease and chronic lower respiratory tract disease – for example, geriatric terrier breeds with mitral valve disease and concurrent bronchomalacia1.
This article will cover the role of bronchoscopy and bronchoalveolar lavage (BAL) in obtaining a definitive diagnosis in dogs with lower respiratory tract disease.
Bronchoscopy is a useful diagnostic technique to investigate lower respiratory disease, as aforementioned. It should be performed as the final step in the diagnostic pathway once a detailed history, complete physical examination, biochemistry, haematology, faecal parasitology and appropriate imaging have been performed.
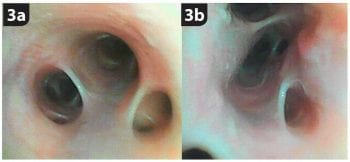
Bronchoscopy itself is rarely definitively diagnostic or therapeutic, except for airway foreign body identification and removal2 (Figure 2). It does, however, help to further characterise the disease process by identifying pathological changes that are not readily identifiable by other imaging modalities, such as dynamic airway collapse due to bronchomalacia3,4 (Figure 3) or bronchiectasis5.
Bronchoscopy does prove invaluable in allowing sampling of the lower airway secretions, which is often essential to achieve a definitive diagnosis in patients with lower respiratory tract disease.
The few absolute contraindications to performing bronchoscopy are:
Patient factors that are not necessarily contraindications, but do carry increased risk of complications, are:
Depending on the patient, a variety of endoscopes can be used for bronchoscopy. A flexible endoscope is required to allow a thorough examination of lower airways.
A bronchoscope is useful in small patients. They come in a range of diameters, from 2.5mm to 5mm; however, their length (50cm to 60cm) can be limiting in the examination of the lower airways in larger patients. The bronchoscope tip only deflects in two directions – up and down – so the bronchoscope is twisted from the handpiece, as required, to direct the tip into smaller airways.
Gastroscopes are useful in larger dogs. The sizes range from 6mm to 8mm – with a length of between 107cm and 160cm – and the tip deflects in four directions. The instrument channel of the endoscope allows passage of instruments (such as aspiration catheters), saline and oxygen.
The endoscope should be as sterile as possible when performing bronchoscopy and BAL to prevent contamination of the airways and collected samples.
The bronchoscope should be cold sterilised – with a disinfectant-detergent cleaning solution – before and after bronchoscopy, then the sampling channel thoroughly flushed with sterile saline before use in the patient.
Suctioning air through the instrument channel after cleaning can prevent residual moisture that could facilitate bacterial colonisation. Additionally, intermittent (monthly) ethylene oxide sterilisation should be considered to prevent bacterial colonisation.
General anaesthesia is required to prevent coughing, laryngospasm, sneezing or gagging during bronchoscopy. The anaesthetic protocol depends on the patient and should be tailored to reduce cardiopulmonary depression.
Prior to anaesthesia, the patient should be preoxygenated for at least five minutes. Premedication with terbutaline (0.01mg/kg SC 30 minutes prior to the procedure) – a bronchodilator – is useful to reduce bronchospasm and improve oxygenation in dogs with a history of airway collapse, bronchospasm or obstructive airway disease.
Typically, patients are premedicated with low-dose acepromazine with an opioid, followed by induction with alfaxalone or propofol. Once anaesthetised, the patient is positioned in sternal recumbency with the head elevated and neck extended (Figure 4).
It is important to protect the endoscope from the patient biting it during examination, with the use of a spring-loaded dental gag. In smaller patients, the use of a roll of tape or piece of rubber tubing could be considered, as spring-loaded dental gags have been associated with cortical blindness due to compromise of the maxillary artery blood supply to the brain and retina in cats6.
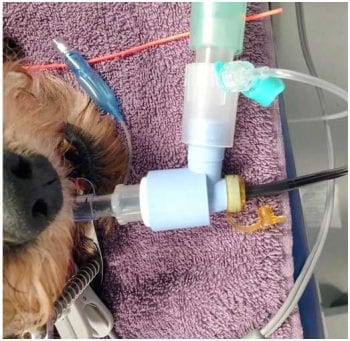
Anaesthesia is typically maintained using volatile gaseous anaesthesia with isoflurane or sevoflurane. A swivel tip T-adaptor with a rubber valve at the top of the port, which opens enough to allow the endoscope into the airway without allowing gas to escape into the environment, should connect the endotracheal (ET) tube to the anaesthetic circuit (Figure 5). This allows maintenance of oxygen and anaesthetic gas flow to the patient.
If the endoscope is to be placed within the ET tube, it is important to test the ease of movement of the scope within the ET tube before intubating the patient.
Smaller patients, in which the ET tube is too small to accommodate the bronchoscope, are maintained with total IV anaesthesia (TIVA). Oxygen can be delivered to these patients either through a soft catheter passed into the trachea alongside the bronchoscope (for example, a 3.5F sterile feeding tube), or through the instrument channel of the endoscope.
Oxygen flow rates of between 1L/min and 3L/min are safe – any higher and reports exist of damage to the small airways through overinflation, ruptured alveoli and pneumothorax7.
If oxygen is constantly being delivered through the channel of the endoscope, carbon dioxide cannot escape – so it is important to remove the endoscope from the airways in smaller patients every 30 seconds to 50 seconds to prevent hypercapnia and overinflation of the lungs8.
It is important to monitor electrocardiography (ECG), pulse oximetry (SpO2), end-tidal carbon dioxide (ETCO2) and cardiovascular parameters before, during and after bronchoscopy.
SpO2 should be maintained at higher than 95% during the procedure. The procedure should be terminated if SpO2 drops to lower than 90% for more than a few minutes or in the case of a bradycardia unresponsive to standard interventions9.
Rapid respiratory or cardiovascular decompensation can occur during or after the procedure. Patients should be closely monitored – a crash kit should be stocked with routine emergency drugs, with cardiopulmonary resuscitation equipment readily available.
After BAL or bronchoscopy, the patient should remain intubated on 100% oxygen for 10 minutes to resolve any hypoxaemia and be monitored closely on recovery, as they can deteriorate rapidly.
Monitoring should include, at a minimum: SpO2; temperature; respiratory rate, depth and effort; heart rate and rhythm; ETCO2; and blood pressure. Crackles over the lung fields may be auscultated for up to 24 hours post-BAL.
A standard technique should be employed for examining the airways of every patient, taking into account the relevant lung and airway anatomy (Figure 6).
Begin by examining the larynx, under a light plane of anaesthesia, to assess movement of the larynx during respiration. Then progress into the trachea under a deeper plane of anaesthesia, noting the dorsal tracheal membrane connecting the C-shaped cartilages.
Once at the carina, it can be helpful to instil 0.2% lidocaine solution to abate coughing during examination10.
From here, move forward to the mainstem bronchi – the right mainstem bronchus will be on the left side of screen and usually straight ahead. The left mainstem bronchus branches at an angle to the right. Follow each branch systematically, examining the entrances to the smaller airways.
Visualisation of the airways allows assessment of the following gross pathology: haemorrhage, mucus secretion, inflammation, bronchoconstriction, nodules, masses, and static or dynamic airway collapse.
Examination and diagnostic procedures should not take longer than between 7 minutes and 10 minutes to reduce the risk of complications.
After bronchoscopic evaluation, the endoscope is slowly advanced into the lower airways until the tip of the bronchoscope is gently wedged in a terminal bronchus – taking note of its position within the lung lobes.
The flat end of the scope should not be pushed against the bronchial mucosa, as this can block the instrument channel opening and result in poor sample retrieval.
To allow for collection of a diagnostic sample, the edges of the bronchoscope tip should form a seal with the surrounding mucosa allowing aspiration of lavage fluid and preventing aspiration of air around the edges of the insertion tube. A BAL catheter is then passed through the instrument channel until it is just beyond the tip of the bronchoscope within the airway.
Sterile saline, warmed to 37°C, is then infused into the BAL catheter or instrument channel, followed by 3ml to 5ml of air. Saline is rapidly absorbed in the lungs, so aspiration should begin no later than between 20 seconds and 30 seconds after instilling the saline9,10.
The time between infusing the saline and aspiration is known as the dwell time. The volume to infuse varies between sources, but the author recommends using 20ml boluses in dogs heavier than 10kg and 5ml to 10ml boluses in dogs lighter than 10kg.
It is recommended up to two to three boluses are performed at each site for collection of an adequate sample. Some authors recommend discarding the first bolus, as it can contain the most contamination from larger airways7.
A suction pump – in combination with a mucus trap (Figure 7) – can be used in preference to manual aspiration, as it has been shown to retrieve significantly more BAL fluid in one study10. Coupage should also be considered to aid sample retrieval.
Samples are obtained from at least two lung lobes to increase the chance of obtaining a representative sample.
Excessive negative pressure during aspiration can cause airway collapse or haemorrhage. If negative pressure is noted, stop suctioning and gently reposition the bronchoscope/catheter slightly without removing it from the airway.
Cytology:
PCRs:
An ideal sample should retrieve between 40% and 90% of the fluid instilled, have some mucoid material within it, and have foam on top of the aspirate – indicating the sampling of alveolar surfactant (Figure 8). Cytologically, these samples should contain alveolar macrophages and contain few respiratory epithelial cells (indicating sampling from the upper airways). The sample is transferred into ethylenediaminetetraacetic acid tubes for cytology, and plain tubes for cultures and PCRs (Panel 2).
Cultures are recommended, as many cases of lower respiratory tract disease have bacterial involvement (often secondary infection) and multiple species are frequently identified from each sample11.
PCR is recommended to increase the sensitivity of testing for certain organisms – as demonstrated in a case series of Angiostrongylus vasorum-infected dogs, in which all seven dogs were positive on BAL PCR, but only two were positive on the rapid assay12.
It is also recommended to make fresh air-dried, unstained smears of any flocculent/mucoid material to be submitted to the external laboratory for cytology, due to the fragile nature of the cells.
A “blind” BAL can be performed if an appropriate endoscope is not available. Patient preparation is the same as with bronchoscopy, and an 8F urinary catheter is used for aspiration. The catheter is cautiously advanced into the lower airways, through the ET tube, until resistance is felt – indicating it has lodged in a terminal bronchus. Sterile saline is then instilled, aspirated and handled as aforementioned.
The complications of BAL are: