3 Dec 2024
Joana Rijo, Pieter Defauw and Renata Stavinohova discuss how calcium deficiency can lead to such eye problems in canine and feline patients.

Bilateral hypocalcaemic cataract in a European cat. Image: Charles Cassagnes
Calcium has an important role in cell signalling and skeletal mineralisation. Its homeostasis is regulated by parathyroid hormone, calcitriol and calcitonin. In circulation, serum calcium is found in three distinct fractions: protein-bound, anion-complexed or chelated, and the biologically active form as ionised or free calcium.
Normal homeostasis mechanisms keep the serum calcium levels within a normal range; however, disruptions in this homeostasis can lead to hypocalcaemia, which can manifest as neurological, cardiovascular and ocular signs. This article reviews the mechanisms, differential diagnoses and clinical manifestations associated with hypocalcaemia, with a particular emphasis on ocular manifestations such as cataracts and prolapsed nictitating membranes. The lens transparency in dogs and cats with hypocalcaemia is affected by defects in the active cation transport and membrane damage.
Insights into the mechanisms underlying cataract formation – especially with hypocalcaemia – brings light on potential therapeutic strategies to slow its progression. Overall, this review provides valuable knowledge of the impact of hypocalcaemia on ocular health, emphasising the importance of early recognition and management to prevent irreversible ocular complications.
Keywords: calcium, hypocalcaemia, cataracts, prolapsed nictitating membrane, ocular manifestations
Calcium (Ca2+) is an essential mineral with an important role in cell signalling and skeletal mineralisation. Its regulation is crucial for normal cell function, membrane stability, neural transmission, bone structure and blood coagulation1,2.
It is believed that more than 99% of Ca2+ is stored in bone as hydroxyapatite, which provides both skeletal strength and a reservoir for Ca2+ to be released into blood circulation2,3. In circulation, total Ca2+ (tCa2+) is found in three distinct fractions that account for different percentages of the serum Ca2+ – protein-bound (35% to 40%), anion-complexed or chelated (8% to 10%), and the biologically active form ionised or free Ca2+ (50% to 55%).
The primary calcium-binding proteins in serum are albumin (around 80%) and globulin (around 20%), whereas calmodulin is the primary calcium-binding protein in the cells. While these proteins cannot be used by tissues, the chelated fraction allows Ca2+ to be absorbed by multiple tissues and transported throughout the body, while ionised calcium (iCa2+) is used by the body to maintain physiologic functions2-4.
Parathyroid hormone (PTH), calcitriol (1,25-dihydroxyvitamin D3) and calcitonin are the main regulators of Ca2+ homeostasis, affecting predominantly the gastrointestinal tract, kidneys and bones. Secretion of PTH by the chief cells of the parathyroid gland occurs when concentration of iCa2+ is low, which increases serum Ca2+ by mobilising Ca2+ from the bones, increasing renal tubular resorption and consequently stimulating calcitriol synthesis.
Calcitriol, which is the most active form of vitamin D, increases serum Ca2+ by increasing intestinal absorption, facilitating PTH-induced bone resorption and increasing renal tubular resorption. Calcitonin is produced in the thyroid in response to elevated serum Ca2+ levels; it inhibits osteoclastic bone resorption activity, and reduces renal tubular resorption2,5. Normal homeostasis mechanisms carry on the serum Ca2+ levels within a normal range; however, in cases of disruption this can lead to hypocalcaemia or hypercalcaemia5.
Hypocalcaemia is an electrolyte imbalance characterised by abnormal low levels of serum Ca2+ that frequently occurs due to different disorders, and can be temporary, reversible, chronic or even lifelong, depending from the underlying cause1.
Total hypocalcaemia is considered when serum tCa2+ is less than 9mg/dL or less than 2.2mmol/L in dogs, and less than 8mg/dL or less than 2mmol/L in cats, while ionised hypocalcaemia is considered when serum iCa2+ is less than 5mg/dL or less than 1.2mmol/L in dogs, and less than 4.5mg/dL or less than 1.1 mmol/L in cats2.
Possible underlying conditions associated with hypocalcaemia are presented in Panel 1 – the most common clinical signs that develop and those that are directly attributable to a hypocalcaemia-induced increase in neuronal excitability.
For example, this could include behavioural changes such as restlessness, aggression, hypersensitivity and disorientation. In addition, syncope or seizures, focal muscle twitching (especially the aural and facial muscles), rigidity or muscle cramping, stiff gait and tetany can occur.
Additional physical examination findings may include hyperthermia, distended and tense abdomen, cataracts, raised nictitating membranes (only reported in cats), cardiac abnormalities such as congestive heart failure, and tachyarrhythmias with soft heart sounds and refractory hypotension6. Moreover, hypocalcaemia may contribute to sepsis-associated myocardial dysfunction.
Early indicators of hypocalcaemia include lethargy, anorexia, intense facial rubbing and panting. If severe, hypocalcaemia may result in coagulation abnormalities2,7,8. Ionised hypocalcaemia is also associated with mortality in both dogs and cats, which is reflected by the length of hospitalisation in the intensive care unit and is an indicator of poor prognosis in cats when iCa2+ concentrations do not normalise during hospitalisation7.
Humans may also experience numbness and tingling, bronchospasm and wheezing, laryngospasm and dysphagia, irritability, depression, fatigue and if hypocalcaemia becomes chronic, it may result in coarse hair, fragile nails, psoriasis, dry skin and poor dental health4.
Hypocalcaemia should be confirmed before performing any diagnostic tests to identify the cause, preferably by measuring the serum iCa2+ concentration. The history, physical examination findings, haematology, serum biochemistry panel and urinalysis usually provide indications of the underlying diagnosis8. Causes of hypocalcaemia in dogs and cats are summarised in Panel 1.
The most common causes are non-pathologic, temporary or insignificant causes, in which iCa2+ concentrations are typically mildly decreased. However, critical illness and renal disease are the most common pathological causes in both dogs and cats, including urethral obstruction in cats.
Cases of moderate to severe hypocalcaemia, hypoparathyroidism and eclampsia are also considered common in dogs, whereas in cats soft tissue trauma is also considered as a common cause7.
The mechanisms that underlie the association between critical illness and hypocalcaemia, such as in case of septic peritonitis, are poorly understood and most likely multifactorial. Nevertheless, some theories include changes in PTH levels, vitamin D deficiency, hypomagnesaemia and accumulation of Ca2+ in tissues.
In acute kidney disease, the hypocalcaemia is hypothesised to result secondary to decreased glomerular filtration rate that causes hyperphosphataemia and consequently increased binding between Ca2+ and phosphate. When urethral obstruction occurs, phosphate is retained secondarily to the obstruction, PTH resistance and acid-base alterations could result in hypocalcaemia7,8.
With chronic kidney disease, mild ionised hypocalcaemia is often present; however, concurrent pancreatitis or acute kidney disease could also be the cause. Hypocalcaemia in kidney disease can also occur as a consequence of the decrease in calcitriol production once inactive vitamin D is absorbed in the intestine. It is transported through the liver, and then to the kidney, where it is hydroxylated to the active metabolite 1,25-dihydroxyvitamin D (calcitriol). This lack of calcitriol can also lead to reduced intestinal absorption of calcium2,7,8. Additionally, osmotic diuresis and supplementation of bicarbonate, or potassium phosphate, may cause this reduction in iCa2+ levels7,8.
The lens is a transparent, avascular structure that focuses light on to the retina and is supported by zonules that extend from the ciliary body epithelium, and attach around the lens capsule at the equator. It is further stabilised posteriorly within a shallow depression in the anterior vitreous, with the iris resting against its front surface.
Enclosed in a transparent capsule made primarily of type IV collagen, the lens grows throughout life, with new fibres added to the cortex, compressing older ones and reducing accommodation ability with age. The lens maintains transparency through fibres rich in crystallines, and is composed of a lens capsule, an anterior epithelium, a nucleus and a cortex9.
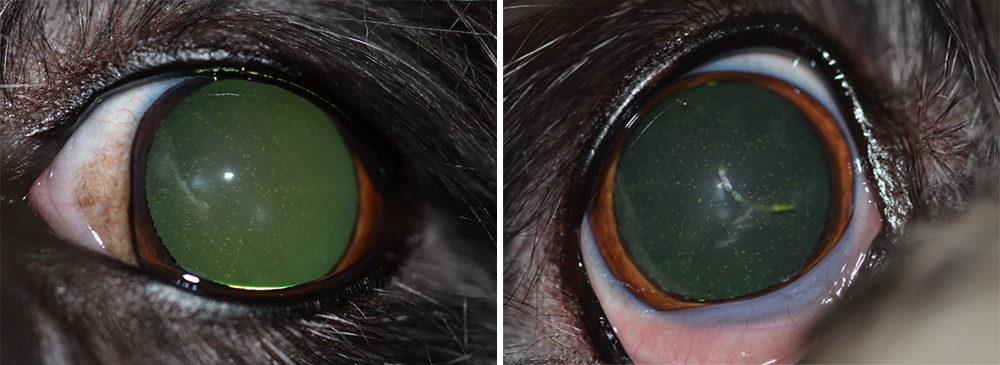
Figure 1. Bilateral hypocalcaemic cataracts in a bichon frise. Images: Charles Cassagnes
In dogs and cats, the lens measures about 0.5ml in volume, with slight variations in dimensions, with anteroposterior axis about 7mm in dogs and 7.5mm to 7.8mm in cats, and diameter about 10mm in dogs and 9mm to 10.4mm in cats.
The lens relies on aqueous humour for its metabolic needs due to the lack of a direct blood supply, and the lens epithelium is responsible for producing energy for inorganic ions and amino acids transport, and for protein synthesis.
Consequently, cataractogenesis may occur due to this reliance, with contributing factors including Na+-K+-ATPase deficiencies affecting ion balance, inadequate antioxidants failing to manage oxidative stress, and excessive glucose conversion to sorbitol causing lens swelling9.
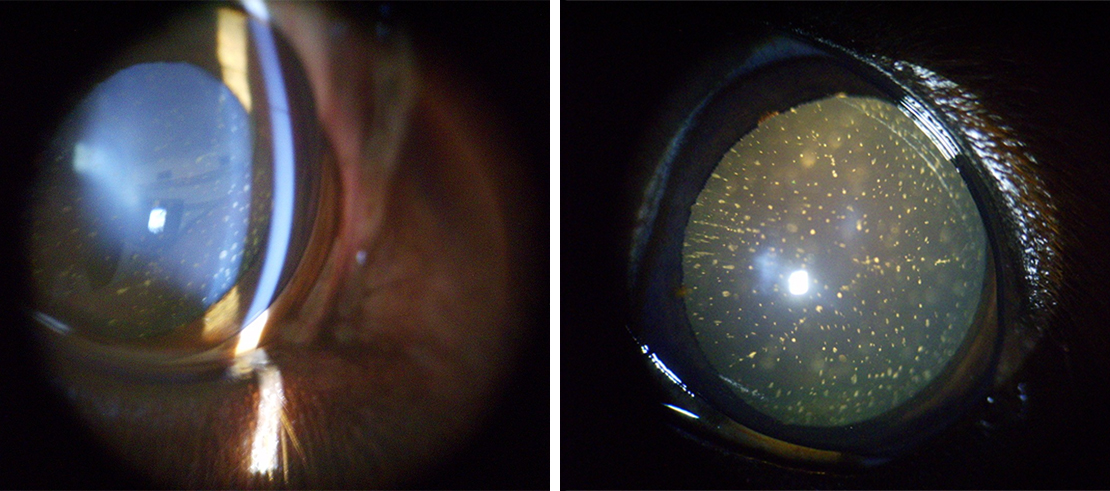
Figure 2. Bilateral hypocalcaemic cataracts in an English cocker spaniel. Images: Charles Cassagnes.
When analysing the concentration of Ca2+ in healthy canine lenses compared to lenses with cataracts, it was concluded that Ca2+ homeostasis in the lens is an essential factor for maintaining lens transparency – healthy lenses presented with a homogenous distribution and low concentrations of Ca2+. In immature cataracts, there was a slight increase in Ca2+ concentrations though Ca2+ remained evenly distributed in the cortex region, with a slight increase in the nucleus.
In mature cataracts, concentrations of Ca2+ were higher and presented with a heterogeneous distribution, losing the even distribution observed in healthier states. In hypermature cataracts, Ca2+ levels were relatively higher around the nucleus in the cortex region11.
Among dogs, cataracts is one of the most common ocular abnormalities (Figures 1 and 2), although their prevalence in cats is lower (Figure 3).
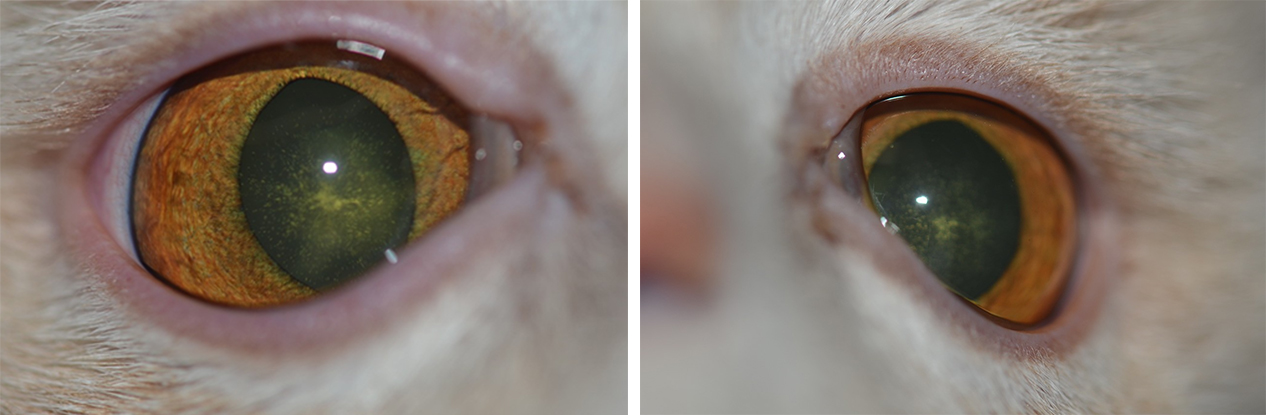
Figure 3. Bilateral hypocalcaemic cataracts in a European cat. Images: Charles Cassagnes.
Five different stages of cataract progression exist – incipient, immature, mature, hypermature and Morgagnian cataract. Cataracts can remain stable or can mature into complete cortical cataracts, causing partial or total opacity of the lens and/or the capsule, and may lead to blindness. At this point, the ocular fundus is obstructed during ophthalmic examinations and is not possible to evaluate9-11-13.
When secondary to hypocalcaemia, cataracts may develop as a consequence both in dogs and cats (Panel 2). Cataracts associated with hypocalcaemia have been widely reported in humans (Panel 3).
Hypocalcaemic cataracts in dogs and cats are multifocal, and punctate-to-linear opacities in the anterior and posterior cortex of the lens lying beneath the lens capsule by a zone of clear lens or coalescing lamellar cortical opacities (like a “field of stars”), which usually is bilaterally symmetric. Lenticular opacities can appear at various levels in the cortex and may represent different phases of hypocalcaemia, being the appearance of cataracts, which is very suggestive of hypocalcaemia.
The degree and duration of hypocalcaemia required to produce cataracts are unknown, but the development is suspected to be related to serum and aqueous humour Ca2+ levels rather than the duration of the disease.
The mechanisms behind the development of these pathologic changes are not completely understood – nevertheless; they are thought to be related to hypocalcaemia-associated defects in the active cation transport mechanism of the lens epithelium.
This subsequently leads to an increase in sodium content and a loss of lens potassium, causing an osmotic imbalance, resulting in lens fibre swelling and membrane damage.
Treatment of the underlying hypocalcaemic disease is expected to halt cataract progression. However, correction of hypocalcaemia will not reverse existing lens opacities. No therapeutic strategies have been identified in the literature regarding the trace elements that could contribute to cataracts1,6,9-12.
Hypocalcaemia has been associated with prolapse of the nictitating membranes, especially in cats. This condition has mainly been observed with acute hypocalcaemia, but is not reported with chronic hypocalcaemia. The nictitating membrane, also called the third eyelid, is responsible for protecting the globe, distributing tears, and producing immunoglobulins and part of the precorneal tear film.
When concentrations of serum Ca2+ decrease, the excitability of neuromuscular tissue increases, which is suspected to be the main mechanism that causes prolapse of the third eyelid6,9,14.
Other ocular signs of hypocalcaemia may occur, but the mechanism behind them is not completely understood. These may include papilledaema, optic neuritis, conjunctivitis, keratitis, blepharospasm, loss of eyelashes, strabismus, nystagmus and anisocoria6.
We would like to extend our thanks to Charles Cassagnes for providing the photographs for our article.