14 May 2018
Ellie Mardell explores this respiratory disease in cats, and its management and treatment, focusing on immunotherapy (video content at the foot of the article).

Image: Nataliia Pyzhova / Adobe Stock
The cat with a chronic cough is a familiar presentation in small animal practice.
In addition, a proportion of cats presenting with acute respiratory distress will be diagnosed with lower airway disease, often with a sterile inflammatory component. We broadly treat these patients in a similar manner, with steroids forming the mainstay of therapy, and bronchodilators of particular importance in an acute setting.
What if we had a way to identify an allergic process in some or all of these cats, and optimise management with the use of a desensitising vaccine, effectively curing the patient?
In humans, asthma, chronic bronchitis and chronic obstructive pulmonary disease (COPD) are recognised as separate, but frequently overlapping disease entities.
Chronic bronchitis is defined as mucus hypersecretion with a chronic cough; but strictly, the term does not imply airway obstruction. In both asthma and COPD an underlying chronic inflammatory process exists and resultant obstruction of airflow; however, they have different underlying aetiologies and pathophysiology.
Asthma is characterised by acute, but reversible, bronchoconstriction in hyperreactive airways, usually mediated by eosinophils and often induced by a hypersensitivity response. In contrast, in COPD irreversible bronchial narrowing and mucus overproduction occurs, and, typically, neutrophilic inflammation, often as a result of chronic inhalation of airway irritants, rather than allergens.
In people, peak expiratory flow rates (PEFR) can be easily assessed with a handheld in-clinic meter. People with mild asthma may frequently be treated symptomatically with inhaled bronchodilators, and, sometimes, inhaled steroids, with very little else in terms of prior investigations to confirm the condition. Monitoring response to treatment will be undertaken again with PEFR measurements.
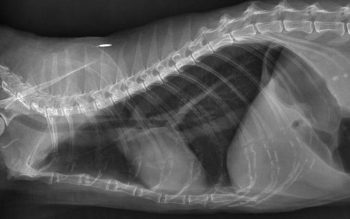
In more severe or equivocal cases, bronchoprovocation studies and airway cytology may be used as confirmatory tests for asthma, and to differentiate between asthma and other lower airway diseases.
Immunotherapy is a treatment option for people with asthma. It may be recommended when it is impossible to avoid a known allergen, when medication is ineffective at controlling the signs (particularly when multi-modal therapy is insufficient), or when signs are present year-round and a desire or necessity to avoid continuous use of medications exists.
People with allergen-induced asthma may have a suspicion of which substances are likely to cause an attack. Skin testing is used to confirm an allergic response to these and to identify other offending agents from a typical range of known potential allergens, such as pollens, animal dander, moulds and house dust mites. Desensitising vaccines are given once to twice weekly for four to six months, with an increasing dose of allergen being given each time, until the optimum dose is achieved. A maintenance phase follows, with this dose being administered less frequently. Treatment usually continues for three to five years, but it is noted, particularly in children, the beneficial effect may persist much longer than this. A reduction in the severity of asthma signs is seen, bronchoreactivity is reduced and steroid medication can be reduced or, occasionally, phased out altogether.
A Cochrane review noted immunotherapy can be as effective as steroid treatment. However, response rates and degree of response do vary quite considerably. Also, allergic reactions to the injections, and even anaphylaxis, are recognised as a significant risk, with just over 10% of patients reported to show a significant systemic side effect. For this reason, immunotherapy is not routinely recommended for all patients, and asthma must be adequately controlled before considering desensitisation to avoid serious exacerbation of respiratory signs following an injection.
Variations on this therapy include peptide immunotherapy, where shorter sections of the allergen protein are administered, which reduces the chance of adverse reactions and sublingual immunotherapy, which has a less certain evidence base, as it has typically been used for less severe asthma.
Whether sterile lower airway diseases in cats exactly parallel those in humans is not clear.
Histopathological specimens of the lungs of affected cats (variously ante or postmortem, depending on the study) do not always correlate well to cytological findings of bronchoalveolar lavage (BAL); this contrasts to the situation in humans. Pathological findings in cats also show some overlap between the conditions that would be categorised separately in people.
Finally, with veterinary patients, investigative techniques are usually somewhat more limited. Access to pulmonary function testing is uncommon in a clinical setting, and its interpretation is not always straightforward, and bronchoprovocation is not considered ethical in client-owned cats, given the potential for fatal bronchospasm to occur.
Terms as specific as “feline asthma” or “chronic bronchitis” may, therefore, be misleading, implying a greater understanding of a feline patient’s disease than is afforded by standard investigative techniques.
Little is known about the initiating causes or exacerbating factors in feline asthma/bronchitis. Genetic predisposition may play a part; Siamese cats appear overrepresented for inflammatory lower airway disease. Asthma can be produced experimentally in cats using dust mite or certain grass allergens, and airway hyperreactivity can be demonstrated in these cats. While possible allergens, such as grasses, pollen, house dust mites, dander and fungal spores, have been, to some extent, evaluated as causes of acute airway spasm in cats, conclusive proof of airway hypersensitivity as a cause of signs in clinical patients, is lacking.
In people, as well as dogs, the predominant cell types in the BAL fluid (BALF) collected from the lungs of normal animals are epithelial cells and alveolar macrophages. Eosinophil numbers are very low and, in humans, an increased number and percentage of eosinophils is considered a hallmark of asthma. In contrast, airway inflammation in COPD is neutrophilic; macrophage numbers are also increased, although proportionally less so.
In BAL samples from healthy cats, again, alveolar macrophages are present in large numbers. However, in most studies, feline BAL samples frequently contained large numbers of eosinophils, and they may even be the predominant BAL cell type. A large proportion of neutrophils may also be present in clinically healthy cats. Finer classification of feline lower airway disease is, therefore, challenging. Crudely, our diagnosis must rely on the presence of clinical signs, demonstration of supportive findings, such as radiographic changes and airway cytology, and exclusion of various infectious (or occasionally neoplastic) causes.
However, a variety of clinical presentations may be appreciated; some cats have a mild but persistent cough for months to years, others rarely cough, but show continuous laboured, wheezy breathing, and a minority of cats lead a normal symptom-free life, but suffer intermittent, sudden onset severe respiratory distress, which, in some cases, may be life-threatening. These latter presentations may be the ones that most closely resemble asthma in people. Interestingly, radiographic changes may sometimes be subtle or lacking in this subset of feline patients, supporting a theory dynamic reversible bronchoconstriction is occurring in response to some stimulus.
Control is generally regarded as good in the majority of cases, and a few studies have demonstrated efficacy of the inhaled glucocorticoids, both in naturally occurring feline asthma and experimental models of the disease. Glucocorticoids or other immunosuppressive treatment are likely to be required life-long however, and usually daily or at least every other day. Also of concern, is one study that demonstrated ongoing bronchial inflammation in some cats, despite a good clinical response (Cocayne et al, 2011). This could potentially mediate ongoing damage to the airways.
An alternative approach would be desensitisation of affected cats, if allergens triggering their asthma could be identified. This has yet to be achieved in a clinical setting, but a small number of studies have evaluated the possibility under experimental conditions, with good results.
More than a decade ago, Reinero et al (2006) demonstrated efficacy of an abbreviated course of immunotherapy (known as rush immunotherapy), given over two days.
Cats in the study were deliberately sensitised to Bermuda grass allergen, such that eosinophils were increased in quantity in its BALF, compared to control cats. A group of sensitised cats were then treated with rush immunotherapy, and several markers, including eosinophils (BALF only), immunoglobulins and cytokines (BALF and serum), were evaluated over time in all cats.
A significant reduction in the eosinophilic inflammation of the airways was detected in the sensitised cats which were treated with immunotherapy.
A range of adverse events were noted following injections, including swelling of the injection site, tachypnoea, pyrexia, vomiting and tachycardia. These were rarely severe, but anaphylaxis was seen (and successfully managed) in one cat. A similar protocol using immunotherapy adjuvanted with an additional DNA molecule showed similar efficacy, but fewer adverse effects.
A third study (Chang et al, 2013) showed the use or oral glucocorticoids may have a negative effect on the response to immunotherapy, but inhaled treatment appeared not to interfere, suggesting signs can be safely controlled with conventional treatment until desensitisation is successful. Finally, presumably in an attempt to mimic sublingual immunotherapy in people, intranasal immunotherapy was trialled in cats with allergen-induced asthma. Efficacy was demonstrated, but the outcome was slightly inferior compared to parenteral immunotherapy.
How could this be applied in our clinical cases? For cats with atopy, allergen detection can be undertaken with either intradermal skin testing (IDST) or by measurement of serum IgE. Whereas some studies have shown a poor correlation between these two methods, a single study evaluating the efficacy of these methods for cats with Bermuda grass allergen or house dust mite allergen deliberately induced airway eosinophilia (Lee-Fowler et al, 2009), therefore showing both methods were able to reliably detect the induced allergic response, with adequate specificity, as long as an enzyme-linked immunosorbent assay method was employed for serum testing. IDST had the slightly better sensitivity.
At first glance, it may seem inexplicable this investigation and treatment modality has not been adopted in feline medicine. However, it is unclear how closely the experimental models of asthma used in the studies mimic our patients in a clinical setting. Experimentally, the allergen is deliberately selected and can be shown to cause sensitisation with disease expressed in the airways. Eosinophilic inflammation is generated in the experimentally sensitised cats; compared to a diversity of BALF findings in our clinical feline “asthmatics”; we are not yet certain allergen-induced disease is a significant problem for even a sub-population of cats. Unfortunately, as yet too little work has been carried out to identify which agents may act as allergens in cats naturally affected with lower airways disease; the main target organ of interest for the characterisation of allergens in allergic disease has been the skin.
One study came a little closer to the mark (Moriello et al, 2007), identifying via IDST, several airborne allergens that caused a positive reaction in cats with respiratory disease, but lacking skin disease, suggesting a possible link between these allergens and a reaction in the respiratory tract. Desensitisation was not attempted, however.
Finally, the fact cats tolerate steroid treatment so well may reduce the drive to seek new treatments. Particularly given with the now quite widespread acceptance of inhaled therapy, side effects of even long-term treatment can be minimised.
In people, the Cochrane review does not show clear evidence of a superior outcome with immunotherapy, compared to conventional steroid treatment of asthma, and the possibility the same might be true for our feline patients may reduce the drive to investigate this option further. Still, in the author’s opinion, it would be an interesting avenue to explore, given it has the potential to both increase our understanding of the pathogenesis of feline lower airway disease and add to our therapeutic armoury in certain cases.