24 Apr 2017
Catherine Bovens discusses various methods of diagnosis and treatment for this common autoimmune disease present in dogs.

Figure 2. A positive saline autoagglutination test, demonstrating macroscopic red blood cell agglutination.
Immune-mediated haemolytic anaemia (IMHA) is one of the most common autoimmune diseases in dogs.
It typically results in severe anaemia that develops acutely and is caused by the production of antibodies targeting red blood cells. These antibodies either cause activation of the complement cascade, resulting in intravascular lysis of red blood cells, or opsonise red blood cells causing phagocytosis by the monocyte-phagocyte system in the liver and spleen.
IMHA is most commonly a primary condition (a primary autoimmune disease without a detectable underlying cause), but is sometimes secondary and triggered by another disease or exposure to drugs or toxins (Panel 1). The disease has a genetic susceptibility component, as evidenced by its high prevalence in certain breeds, such as cocker spaniels.
Diagnosis of IMHA is based on documentation of a regenerative anaemia (absolute reticulocyte count more than 60 × 109/L to 80 × 109/L) with exclusion of external blood loss (exclusion of external bleeding, including haematemesis, melena, haematochezia and haematuria based on history; severe infestation by fleas, ticks and hookworms), exclusion of internal blood loss (absence of effusions on thoracic and abdominal imaging) and exclusion of other causes of haemolysis (Panel 2).
Importantly, chronic low-grade gastrointestinal bleeding may not be apparent externally; if the anaemia is poorly regenerative – especially if microcytosis and/or hypochromasia are present (consistent with iron deficiency), or if gastrointestinal signs are present – chronic gastrointestinal bleeding must be considered a possible differential diagnosis.
Some dogs with IMHA have a non-regenerative anaemia. This can be due either to detection of IMHA before a regenerative bone marrow response develops (this typically takes three to five days) or immune-mediated disease directed against red blood cell precursors in the bone marrow.
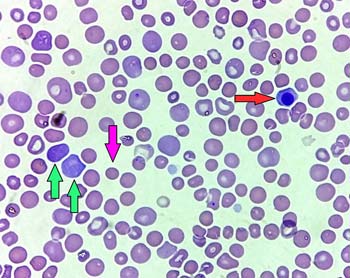
The presence of spherocytes on blood smear is typical of IMHA, although they may also rarely occur in other conditions. Spherocytes are red blood cells that have lost their characteristic shape due to damage from the immune system and have become spherical, causing a small size and lack of central pallor on smear (Figure 1).
A positive saline autoagglutination test indicates the red blood cells have surface antibodies and is consistent with IMHA. To perform a saline autoagglutination test, a drop of fresh blood is mixed with a drop of saline on a glass slide.
If visible agglutination occurs (Figure 2), the slide should be checked under the microscope to make sure true agglutination is present, rather than red cell rouleaux, which can be normal. A negative saline autoagglutination test does not exclude IMHA; in such a case, a Coombs test can be performed. A positive Coombs test is consistent with the presence of antibodies on the red blood cells and IMHA.
Importantly, a positive saline autoagglutination test or Coombs test is indicative of IMHA, but does not tell us if the IMHA is primary or secondary to another underlying condition.
Additional findings that support the diagnosis of IMHA are the presence of haemoglobinuria, haemoglobinaemia and/or an elevated serum total bilirubin.
Once the diagnosis of IMHA is confirmed, further investigation is needed to exclude a possible underlying cause (Panel 1).
This is important as any underlying cause needs to be addressed for successful treatment. Investigation should include haematology (with smear examination) and serum biochemistry, urine analysis and culture, thoracic imaging (radiographs or CT scan), abdominal ultrasonography (recommended over abdominal CT scan as ultrasonography is more sensitive for gastrointestinal lesions that may cause anaemia from bleeding), and testing for possible infections depending on geographical location.
In cases of IMHA, haematology frequently reveals neutrophilia, often with a left shift, indicative of an active bone marrow regenerative response; this should not be mistaken for a sign of infection. Smear examination is recommended to assess platelet numbers, as automated haematology is often unreliable for this. A mild thrombocytopenia may indicate secondary thromboembolic disease. A severe thrombocytopenia is consistent with Evans syndrome, where the platelets are also targeted by the immune system.
Serum biochemistry is usually normal or shows changes secondary to dehydration and/or tissue hypoxia. Increased liver enzymes are common due to liver hypoxia, but other hepatopathies must be excluded. Any decreases in serum protein may indicate blood loss or gastrointestinal disease, and reassessment of the diagnosis of IMHA is recommended. Urine analysis may reveal a secondary proteinuria. In cases of primary IMHA, imaging is usually normal.
In the UK, the epidemiology of tick-borne diseases is expected to change as more pets travel abroad and as species of ticks previously absent from the UK become endemic, probably linked with climate change. Infection with Babesia species has been reported in ticks collected from dogs in the UK, and clinical babesiosis has been reported in several dogs with no history of travel outside the UK1-3. Based on this, blood PCR for Babesia species is recommended in dogs with IMHA in the UK – particularly in Essex. Testing for haemotropic Mycoplasma species is recommended in dogs that are immunosuppressed or had a previous splenectomy.
The mainstay of treatment for primary IMHA is the administration of immunosuppressive medication. Steroids are typically used at an immunosuppressive dose; the author usually administers dexamethasone 0.3mg/kg IV once daily until the patient is stable, then changes to oral prednisolone 1mg/kg twice daily by mouth.
Stable patients can be started on oral prednisolone without first receiving dexamethasone. Higher doses of prednisolone have not been shown to be more effective and increase the risks of side effects, including gastrointestinal ulceration.
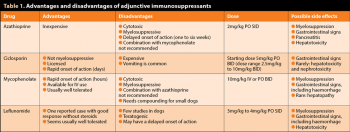
Many dogs respond well to prednisolone alone for immunosuppression, and no evidence exists adding a second immunosuppressant is beneficial4. Adjunction of a second immunosuppressant is recommended in patients where a high dose of prednisolone is likely to cause severe side effects (particularly large dogs), in severe cases (particularly with severe intravascular haemolysis), or if a poor response to prednisolone is seen after three to five days of treatment. Medications most commonly used include azathioprine, ciclosporin, mycophenolate and leflunomide. No published clinical trials provide evidence on which drug is most effective. Cyclophosphamide is not recommended. See Table 1 for the advantages and disadvantages of each drug.
Azathioprine, mycophenolate and leflunomide have the disadvantage of being cytotoxic drugs, requiring handling precautions for both the drug (tablets should not be split or crushed, capsules should not be opened and the medication should be handled with gloves) and for the pet while on the medication (contact with children or pregnant women is not recommended and body secretions should be handled with gloves).
Azathioprine is the most commonly used drug as it is inexpensive, but takes longer to be effective (at least 7 to 14 days, up to 6 weeks), so is less suitable if the response to prednisolone has been poor and rapid immunosuppression is needed. If an appropriate dose cannot be obtained with the existing tablet sizes, it can be reformulated, or used every other day or alternating different doses on different days so the average dose is 2mg/kg/day.
Ciclosporin is not cytotoxic or myelosuppressive, is licensed for dogs and has a more rapid onset of efficacy than azathioprine in most cases (within a week), although its half-life has a large individual variability, making the onset of efficacy and required dose unpredictable. It is very expensive. If using a generic version (not recommended, due to the cascade), a micro-emulsified version of ciclosporin should be used.
Twice-daily administration is required for immunosuppression and the drug should be given on an empty stomach to increase absorption. Vomiting is a common side effect and usually resolves after a few days of treatment.
To reduce vomiting, options include giving the drug with food for a few days initially; administering an antiemetic prior to administration; and/or freezing the capsules prior to administration, which is anecdotally reported as effective – although the effect of freezing on absorption and efficacy have not been fully determined.
If a poor response to treatment is seen, blood levels of ciclosporin can be measured to determine if a sufficient amount of the drug is being absorbed. Trough blood levels (12 hours post-administration) have been used routinely, with a recommended target of 500ng/ml. However, peak blood levels (two hours post-administration) may correlate better with immunosuppressive effects5. Although ideal peak ciclosporin concentrations have not been published in dogs, a target peak concentration of 800ng/ml to 1,400ng/ml has been suggested5.
Mycophenolate is available in an injectable formulation for IV use, which can be helpful in severe cases where rapid immunosuppression is necessary or in critically ill patients that cannot be medicated orally. It induces immunosuppression more rapidly than ciclosporin or azathioprine, even if administered orally (within hours). In one retrospective study, mycophenolate was as effective as azathioprine or ciclosporin6. Available oral capsule sizes are suitable for large dogs and compounding is required for small dogs. While an oral suspension is available, the author would not recommend using it as it makes containment of this cytotoxic medication difficult and human exposure more likely.
Leflunomide has been used in only a limited number of published dogs so far, and very few cases of IMHA. It was effective in a case of Evans’ syndrome in a diabetic dog that did not receive glucocorticoids7.
If the author needs an adjunctive immunosuppressant, an azathioprine is used in a stable dog without history of pancreatitis or liver disease where the author might want to taper the prednisolone dose more rapidly; mycophenolate in a severe or unstable case where rapid immunosuppression is sought; and ciclosporin as a non-cytotoxic medication for dogs living with children or pregnant women, or in an unstable case if mycophenolate is not available.
It should be noted all immunosuppressants increase the risk of infections and this risk increases if several agents are used concurrently. These medications are also likely to increase the risk of future neoplasias – particularly lymphoma.
An IV infusion of human immunoglobulin produces rapid immunosuppression. A prospective study showed no benefit when human IV immunoglobulin was given to dogs with IMHA in addition to glucocorticoids8. It may have a role when rapid stabilisation is needed in severe cases, but no evidence exists of long-term benefit. It is not readily available, and use in dogs of a blood product obtained from human donations and also needed for human treatment raises ethical questions. It may have procoagulant effects, which may be detrimental8.
Splenectomy can be considered in cases refractory to medical immunosuppression, as part of the pathophysiology of IMHA involves antibody-mediated destruction of red blood cells by the spleen. In humans with IMHA, splenectomy is commonly reported and usually performed as a second line of treatment after a steroid trial.
A retrospective study evaluated 10 dogs with splenectomy as part of their early treatment for IMHA, and reported a decrease in transfusion requirements and an increase in haematocrit9. Potential complications include increased susceptibility to infections, bleeding, thromboembolism, and general risk associated with surgery and anaesthesia. Several forms of novel immunotherapy are being researched that are intended to re-establish tolerance of self-antigens rather than broadly suppressing the immune system. Such therapies will, hopefully, allow control of IMHA while still permitting normal responses to pathogens.
In addition to immunosuppressive medication, medication with anticoagulants is recommended to prevent thromboembolism. Thromboembolism is a common and, sometimes, life-threatening complication of IMHA. Although the prevalence of thromboembolism is estimated at 30% to 50% clinically10, necropsy studies suggest it may be higher.
Pulmonary thromboembolism is most commonly recognised clinically. Emboli can also involve many other organs, including the brain, heart, liver, spleen and kidneys. Anti-platelet agents (aspirin or clopidogrel) are the most commonly used anticoagulants for IMHA; heparin is also sometimes used. The optimal drug and dose have not been determined.
The use of anti-platelet agents is only recommended in dogs with a normal or only mildly decreased platelet count. The author uses a low dose of aspirin in IMHA cases (1mg/kg once daily by mouth) – a dose of 5mg/kg once daily has also been suggested10.
A higher aspirin dose may lead to gastrointestinal side effects. Dispersible aspirin can be used for accurate dosage (disperse one tablet with 10ml of tap water, give the required amount and discard the rest). Clopidogrel can also be used instead of – or in addition to – aspirin, but no evidence exists it is more effective than aspirin or giving both agents together is more effective.
Other supportive treatments as needed may include gastroprotectants, IV fluids, blood transfusions and nutritional support. Transfusions of packed red blood cells are preferred over whole blood transfusions, if possible. It is preferable to administer dog erythrocyte antigens (DEA) 1.1 negative blood to negative donors to reduce the risks of future transfusion reactions, although a single transfusion of DEA 1.1 positive blood in a negative donor is unlikely to cause a reaction.
The efficacy of treatment can be monitored with the haematocrit/PCV, saline autoagglutination and blood smears to detect spherocytes.
Once treatment succeeds and a stable PCV more than or equal to 30% is obtained, the author continues the immunosuppressive medications at the full dose for four weeks, if they are tolerated, then tapers them gradually. Anti-platelet agents can be discontinued once no signs of haemolysis are seen, usually after two to four weeks.
While on immunosuppressants, a mild anaemia of chronic disease will persist in many dogs, usually with a PCV around 30% to 32%. This does not mean treatment is ineffective as long as the PCV is stable and no other signs of haemolysis present. If several immunosuppressants are used, the author recommends tapering the medication causing the most side effects first (usually prednisolone).
The prednisolone dose can be tapered by 20% to 25% every two to three weeks, with haematology and smear examination prior to each dose reduction to monitor for possible relapse. A more rapid tapering increases the likelihood of relapse. To taper cytotoxic medications (as tablets/capsules cannot be split) after prednisolone has been discontinued or reached a low dose, a lower dose formulation is used, if available, then the medication is given less frequently (every two days then every three days, and so on) until a low dose is reached for discontinuation (every four or five days).
Serum biochemistry is recommended regularly to monitor for side effects of treatment, such as hepatotoxicity. The author recommends performing a serum biochemistry after two and four weeks of treatment then every six to eight weeks, if no side effects are seen. Urine culture is recommended every three months during immunosuppression to identify subclinical urinary tract infections.
The prognosis of IMHA is guarded, with reported mortality rates of 30% to 70% within one to two months of diagnosis. Dogs that die from the disease are either euthanised due to refractory anaemia or the cost of treatment and transfusions, or die from tissue hypoxia (causing issues such as hepatic or renal failure) or thromboembolism.
Negative prognostic factors include hyperbilirubinaemia, thrombocytopenia, increased band neutrophils and increased blood urea. Interestingly, the degree of anaemia, magnitude of the reticulocyte response and degree of spherocytosis have not been found to correlate with outcome in most studies. Relapse may occur during or after tapering of the immunosuppressants, and some dogs need long-term medication at a low dose.
While the role of vaccination as a trigger for IMHA remains controversial, vaccination may play a role in some individual dogs. Excessive vaccination should be avoided in dogs having suffered from an autoimmune condition. The author recommends a blood test every two to three years to determine the antibody status against parvovirus, distemper and adenovirus, with revaccination only if the dog is no longer protected and following discussion about possible risks with owners.
Vaccination against kennel cough and leptospirosis are unlikely to be effective for years and no reliable blood test exists to determine immunity status. These vaccines can be considered yearly for dogs at high risk, but with discussion of the possible risks. The author recommends avoiding medications that may trigger IMHA in previous sufferers (Panel 1).