24 Jul 2017
Mike Davies summarises evidence found on the use of these supplements in cats and dogs with this joint disease.

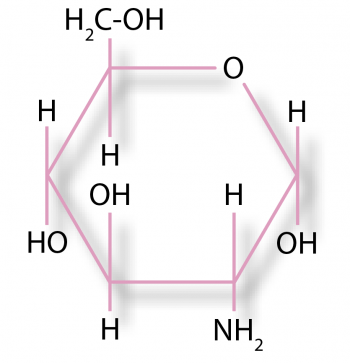
The chemical structure of chondroitin.
Interest has grown in a potential role for glucosamine and chondroitin sulphate in the management of OA in cats and dogs. These are administered as supplements or incorporated into compound foods formulated to aid the management of arthritis.
In this article, scientific evidence is summarised on the use of glucosamine and chondroitin sulphate in OA following a systematic review (unpublished) conducted by the author. Systematic reviews and randomised controlled trials (RCTs) are considered to provide the best level of evidence (levels one and two) compared to observational studies, so are identified in this review.
Glucosamine is an amino sugar and a prominent precursor in the biochemical synthesis of glycosylated proteins and lipids.
It is found in the exoskeletons of arthropods, including crustaceans, and in the cell walls of fungi and higher organisms, and is one of the most abundant monosaccharides.
Glucosamine is available as three salts:
Differences exist between glucosamine salts available in supplements and those present in the body. For example, the sulphate in supplements is present as the anion, whereas in cartilage, it is present as an ester (Hendler and Rorvik, 2001).
Glucosamine is a precursor for several glycosaminoglycans, including hyaluronic acid, keratan sulphate and heparan sulphate, which are major components of cartilage. Another glycosaminoglycan, chondroitin sulphate, contains derivatives of galactosamine, not glucosamine. Glucosamine is not an essential nutrient for dogs or cats as they can synthesise it from other nutrients.
The potential benefit of glucosamine supplementation in OA is based on the observation glycosaminoglycans are important in the maintenance of normal cartilage function. It is produced commercially by the hydrolysis of crustacean exoskeletons or, less commonly, by fermentation grains, such as corn or wheat. Glucosamine supplements are often combined with chondroitin, so they need to be considered together.
Chondroitin is a mucopolysaccharide (proteoglycan) composed of alternating residues of β-D-glucuronic acid and N-acetyl-D-galactosamine sulphate in alternating β(1-3) and β(1-4) linkages. It is present in the ground substance materials in the extracellular matrix of connective tissue, especially blood vessels, lens, cornea, bone, and cartilage.
In developing canine cartilage, chondroitin 6-sulphate and chondroitin 4-sulphate are present, although chondroitin 6-sulphate is most upregulated (Matyas et al, 2004).
In OA, chondroitin sulphate is thought to have anti-inflammatory effects at the chondral and synovial levels. It and its disaccharides reduce nuclear factor κ-light-chain-enhancer of activated B cells nuclear translocation, probably by diminishing extracellular signal-regulated kinase1/2, p38 mitogen-activated protein kinase and c-Jun N-terminal kinase activation (Iovu et al, 2008).
In one study, a group of dogs (n=8/32) given glucosamine and chondroitin before induction of synovitis had significantly less scintigraphic activity in the soft-tissue phase 48 days after joint injection, significantly less uptake in the bone phase 41 and 48 days after joint injection, and significantly lower lameness scores on days 12 to 19, 23 and 24 after injection, compared with other groups (Canapp et al, 1999).
In one study (Adebowale et al, 2002), glucosamine and chondroitin sulphate (measured as total disaccharides) were bioavailable after oral dosing in dogs (n=8). Glucosamine absorption was relatively fast – Cmax 8.95μg/ml and Tmax 1.5 hours after a 1,500mg dose – and the mean bioavailability of glucosamine after single dosing was approximately 12%. The bioavailability of chondroitin sulphate ranged from 4.8% to 5% after single dosing and 200% to 278% on multiple dosing; it accumulates.
In a randomised crossover design, glucosamine supplements were administered daily for eight days, with a one-week washout period between treatments (Maxwell et al, 2016). Relative bioavailabilities were determined for three formulations. The dose-normalised maximum plasma glucosamine concentration was higher for the liquid supplement (5.5μg/ml ± 0.5μg/ml) than for the two tablets (3.1μg/ml ± 0.6μg/ml and 2.1μg/ml ± 0.6μg/mL, P <0.001).
In a study in which radiolabelled chondroitin sulphate was administered orally to dogs (Conte et al, 1995), more than 70% was absorbed. After 24 hours, radioactivity was higher in the intestine, liver, kidneys, synovial fluid and cartilage than in other tissues, and, after 36 hours, compounds were still present in the plasma of dogs.
In a randomised placebo-controlled study involving cats with idiopathic cystitis (n=19), administration of N-acetyl-D-glucosamine (250mg PO q24h) significantly increased plasma glycosaminoglycan concentrations after 21 days of treatment (Panchaphanpong et al, 2011). No studies were found documenting the bioavailability of chondroitin in cats.
Nutrition studies can be difficult to interpret because of the complexities of raw ingredient content – for example, green-lipped mussel (GLM) is a commercially important source of glycosaminoglycans, including glucosamine and chondroitin sulphate, but they also contain omega-3 fatty acids, so any benefits seen following GLM extract administration cannot be attributed to either specific nutrient type.
While a study may be an RCT and regarded as level two evidence, several factors can reduce its power – for example, low numbers of animals included in the study, the use of subjective outcome measures, lack of masking, variability between control and test diets, and the confounding effect of other components in diets that could have an effect on OA.
Previous reviews of nutritional management of OA in dogs used different methodologies and did not agree in their findings.
Aragon et al (2007) considered moderate evidence exists for GLM and a combination of chondroitin sulphate, glucosamine hydrochloride and manganese ascorbate. Sanderson et al (2009) concluded moderate evidence existed for the efficacy of glycosaminoglycan polysulphate and a complete food containing GLM.
In a review by Vandeweerd et al (2012), 16 dog-related papers were included and, after very detailed appraisal, the authors concluded global strength of evidence was low for all the 11 nutraceuticals they examined (including glucosamine and chondroitin with or without manganese and GLM or omega-3 fatty acids), but good evidence existed for some diets supplemented with omega-3 fatty acids.
In a systematic review of glucosamine and chondroitin use in canine OA (Bhathal et al, 2017), the authors concluded: “The available evidence is difficult to interpret due to the use of different manufacturers, salt forms, compositions, sources, strengths, regimens, therapy durations, and combinations of active ingredients.”
In a non-RCT study in arthritic dogs (Gupta et al, 2012; n=7) glucosamine/chondroitin supplementation resulted in a positive clinical effect on subjective pain scores and objective force-plate analysis. However, in an RCT in dogs (Moreau et al, 2003; n=23) in which outcome measures included objective ground reaction forces, and subjective owner and vet assessments, glucosamine/chondroitin supplementation failed to result in any improvement, compared to placebo in dogs with confirmed OA.
In the other RCT (McCarthy et al, 2007; n=35), a positive effect was observed on pain scores, weight bearing and severity of condition, although, in this study, the method of randomisation has been criticised (Kirkby and Lewis, 2012).
In dogs (n=10) with surgically sectioned cruciate ligaments, chondroitin given with hyaluronic acid reduced muscle atrophy and improved lameness (Serrato et al, 2007); however, any observed effects cannot be attributed to the chondroitin alone.
In other studies, chondroitin decreased progression of radiographic signs of OA in dogs (n=10) after 30 and 60 days (Biasi, 2004), reduced proteoglycan loss (n=5; Vieira et al, 2010), and reduced progression of OA and improved limb function faster following cruciate reconstruction (n=20; de Biasi et al, 2005).
However, in an RCT using subjective questionnaire assessment by vets and dog owners, no significant difference was observed between chondroitin supplementation (n=21) and placebo (n=19; Dobenecker et al, 2002).
The use of GLM has been reported in eight studies in dogs. An early study (Korthauer and de la Torre, 1992) reported improvement in lameness and posture in dogs (n=26) given a glycosaminoglycan extract of GLM, and later, in a placebo-controlled study (n=81), subjectively assessed improvements were also reported (Pollard et al, 2006).
In another uncontrolled study (Servet and Marniquet, 2006), 75% of owners and 85% of vets thought a GLM-supplemented diet improved signs of OA based on subjective scoring; however, the effects cannot be attributed to the GLM component as the dogs’ (n=85) baseline rations were different to test food. Feeding a GLM-enriched diet for 30 days to 23 dogs (Rialland et al, 2013) resulted in improved peak vertical force, motor activity and owner assessment compared to a base diet that was, unfortunately, not the same as the test diet; thus, this cannot be attributed to the GLM content.
In two masked RCTs involving cross-breed dogs with OA, the addition of GLM powder to a complete dry ration (n=32) for a six-week period, or feeding a treat containing GLM (n=33), improved overall OA score, joint pain and joint swelling, compared to a control group (Bierer and Bui, 2002; Bui and Bierer, 2003).
A subsequent RCT (Hielm-Bjorkman et al, 2009; n=15) also demonstrated a significant improvement in mobility (assessed by a vet) and pain (on a visual analogue scale) compared to placebo, although alleviation of pain was not as effective as carprofen. However, in another randomised, masked, placebo-controlled study (Dobenecker et al, 2002) GLM extract failed to lead to improvement in signs of OA (n=58) based on subjective owner and vet assessments.
The diets used in three studies demonstrating positive effects of GLM (Servet and Marniquet, 2006; Bierer and Bui, 2002; Rialland et al, 2013) contained other potential modifiers of OA, such as inclusion of omega-3 fatty acids, so their efficacy should be considered to be a function of the whole ration – not a single nutrient type.
In a prospective, blinded, randomised clinical trial (Sul et al, 2014), cats older than eight years, with clinical signs of chronic OA, were assigned to one of two groups with glucosamine/chondroitin or meloxicam administered orally for 70 days, followed by a placebo until day 98. Pre-treatment disease scores were significantly higher in the meloxicam group for owner mobility (p=0.01) and veterinary lameness (p=0.02). Owner mobility scores at day 14 (p=0.01) and day 42 (p=0.002) were significantly improved compared to pre-treatment scores for the meloxicam group, but not for the glucosamine/chondroitin group.
In a randomised, controlled, blinded, parallel group, prospective clinical study (Lascelles et al, 2010), cats (n=40) with no detectable systemic disease, and with at least one appendicular joint with radiographic evidence of degenerative joint disease were included. The primary objective outcome measures indicated activity declined significantly (P=0.001) in the control diet group, but significantly increased (P=0.001) in the test diet group – a diet high in eicosapentaenoic acid and docosahexaenoic acid content and supplemented with GLM extract and glucosamine/chondroitin sulphate.
Sixteen canine studies (seven RCTs and nine non-RCTs) were used, in which the efficacy of glucosamine and/or chondroitin sulphate – given as individual or combined supplements, or as a component of GLM extract or a therapeutic veterinary diet in the management of OA – has been reported. Of these, four out of seven (57%) RCTs and nine out of nine (100%) non-RCTs showed a positive effect.
Unfortunately, the evidence is often weak due to low numbers of animals included in the studies, the use of subjective outcome measures, lack of masking, variability between control and test diets, and the confounding effect of other components in diets that could have an effect on OA.
Only two studies in cats were used, of which one failed to show any clinical benefit. The three systematic reviews disagreed and concluded low, moderate and inconclusive evidence existed to support the use of glucosamine and chondroitin in canine OA.
More RCTs with larger population sizes and objective outcome measures are needed to confirm the true efficacy of glucosamine and chondroitin in the management of canine and feline OA.