13 May 2019
Josie Parker describes her approach to ocular cases, including how to examine for manifestations of systemic disease.

Figure 1. Distant direct ophthalmoscopy. Note the person restraining the patient is positioning the head to look at the examiner by supporting underneath the chin.
Eye cases can pose a difficult clinical conundrum in first opinion practice.
They are often made even more tricky due to limited equipment and inadequate lighting conditions. Battling with the settings on a dusty ophthalmoscope with a dying battery to look at a patient attempting to burrow face first into the chest of a worried owner in a brightly lit consult room springs to mind.

The equipment you will need to perform a basic work-up is as follows:
Equipment such as tonometry and a slit lamp will allow you to get more from your ocular exam, but they are not always available in first opinion practice.

The ophthalmoscope is a versatile and essential piece of equipment, but its full potential is rarely used beyond that of an expensive pen torch. Many brands of ophthalmoscope exist, all with their own quirks and differences, but all are variations on a theme. Taking the time to familiarise yourself with the dials and settings will help you significantly when it comes to your exam.
The ophthalmoscope light is a rheostat, like a dimmer switch, which means you can control the brightness. The temptation is often to turn it to the maximum brightness for the whole of the exam, but it can be useful to dim the intensity when performing distant, direct ophthalmoscopy.
A whole array of light beam shapes is available, but the only two you need are the biggest circular beam and, occasionally, the slit beam setting. The colour filters of most use are the white light and the cobalt blue light for assessing fluorescein uptake.
The number indicates the ophthalmoscope’s refractive power setting, in dioptres, and can be altered by turning the dial on the side.
What do the refractive power values mean? Changing the refractive power of the ophthalmoscope changes the focal point:
The best baseline setting on the ophthalmoscope for you is determined by your own eyes. If you do not require glasses, or are wearing contact lenses, this will be zero.
If you wear glasses, remove them and set the number to your prescription. For short-sighted eyes the setting will be in the negative (red) values; for long-sighted eyes it will be in the positive (black or green) values.
Distant direct ophthalmoscopy assesses the clarity of the visual axis and the symmetry of the pupils (Figure 1).
To perform distant direct ophthalmoscopy:
The number on your ophthalmoscope should be your baseline setting.
You should be able to see the tapetal reflection in both eyes.
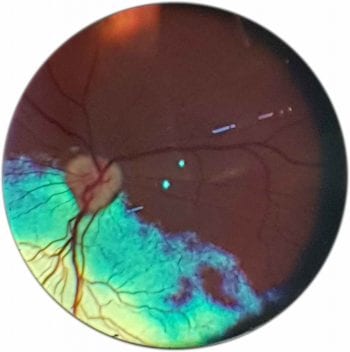
Distant direct ophthalmoscopy helps diagnose opacities in the visual axis, such as cataracts or anisocoria – asymmetrical pupils (Figures 2 and 3).
Ophthalmologists tend to use close direct ophthalmoscopy for in-detail examination of the fundus – preferring to use a slit lamp to examine the anterior structures in the eye. However, direct ophthalmoscopy can be used very effectively for the examination of the whole eye.
Start with your baseline setting on your ophthalmoscope to examine the fundus.
Be aware the brightness of the light decreases with increasing dioptric power (the same as the change in brightness on a light microscope from ×10 to ×40), so increase the brightness if you are struggling to visualise structures at higher settings.
The settings required for a magnified view of the ocular structures vary according to your own eyes, but as a rule of thumb: fundus 0, lens +10, iris +15, cornea +20, and eyelids +25 to +30.
To perform close direct ophthalmoscopy:
The fundus is one of the most exquisitely beautiful structures in the body, but such significant variation exists between individuals, breeds and species that it is very difficult to detect abnormalities. The examination techniques require practice and perseverance to gain any useful diagnostic information.
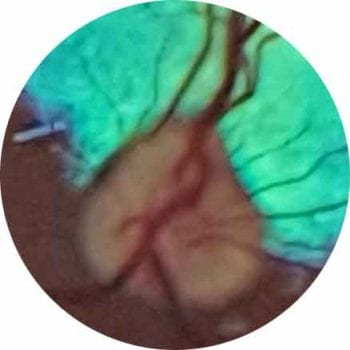
The fundus can be examined using both direct and indirect ophthalmoscopy. Indirect ophthalmoscopy provides an overview of the fundus, but direct ophthalmoscopy enables a more detailed examination of a smaller area. (Figures 5 to 7).
Indirect ophthalmoscopy is one of the most difficult techniques to master, but can be endlessly rewarding as it affords an overview of the fundus, which you can then apply direct ophthalmoscopy to examine areas of interest in fine detail.
The lens creates an upside down, back to front virtual image of the fundus. The standard condensing lens is a 20D lens, although some prefer the 2.2 (22D) pan retinal lens, while others favour 28D or 30D lenses for small animal patients.
Glass lenses produce a sharp image of extremely high quality. They are more expensive – costing around £300 – heavy and easily chipped, and must be handled with a lot of care.
Acrylic lenses are the cheap and cheerful option, costing approximately £40. They are lightweight and resilient enough to withstand being dropped on the floor. They are a great starter lens, as they are versatile and can produce an image of diagnostic quality.
The technique is just as tricky with either glass or acrylic lens – requiring the same amount of practice and perseverance.

If you look at the lens from above, one side is flat and the other is more curved – that is, it has a “belly”. The flatter side should be positioned facing the patient and the belly of the lens towards the examiner – that is belly to belly (Figure 8). Some lenses have a metallic rim to indicate the side that should face the patient (Figure 9), but others are less helpful, and it can be useful to put a dot of paint to quickly identify this side.
A focal light source at moderate to low light intensity such as a transilluminator, pen torch, direct ophthalmoscope or otoscope. The condensing lens does what it says on the tin – it condenses the beam of light as it passes through. The beam emitted from your light source will be intensified, so it is important to use the lowest possible brightness to make it tolerable for your patient.
To perform indirect ophthalmoscopy:

When you move the lens to look around the periphery of the fundus, remember the image is inverted, so when you move the lens medially, the image will move laterally and when you move dorsally, the image will move ventrally and vice versa (Figure 13). This can be very confusing to begin with, but with enough practice, will become second nature.
Tips for success
Within the fundus the tapetum sits dorsally and the optic nerve head sits slightly ventrally. Place the patient on a table and crouch down so you are looking up towards its eye. This positioning will help to initially identify the tapetal reflection, and when you place the lens in front of the eye and stand up slightly, it will aid your view of the ventral fundus and the optic nerve head.
Dilate the pupil with a drop of 1% tropicamide. This will give you a wider aperture to look through and minimise the need to move the lens to look around the periphery of the fundus.
Binocular head sets are often used by ophthalmologists instead of monocular light sources, such as a pen torch. This has the advantage of allowing placement of both hands on the patient to help with positioning of the head.
The light source is positioned between the examiner’s eyes. The head set has a series of mirrors to provide a coaxial light beam in line with each eye, which improves depth perception and can aid the diagnosis of papilloedema, retinal detachment, tumours or other mass lesions, and cupping.
Variations in fundus colour and pigmentation are usually the least clinically significant, but the most distracting findings as they draw the attention of the examiner away from the areas that can actually lead you to a diagnosis (Figure 14).
Always examine both eyes – most normal variations in fundus appearance are roughly symmetrical.
Remember, if you identify an abnormality on indirect ophthalmoscopy, use direct ophthalmoscopy to examine this area in magnified detail.
Photographing the eye is an excellent way to document the initial presentation and progression of a case, but they can also be sent to ophthalmologists for a specialist opinion. For example, Langford Vets runs a free email advice service ([email protected]) and can provide more detailed information for cases with a good quality photograph attached.
1. Clean away significant ocular discharge and fluorescein stain using saline flush.
2. Turn your flash on.
3. Make sure the camera is focused on the area of interest.
4. Take the picture in a dark room.
5. Attract the patient’s attention, so it is looking directly at you.
This article was reviewed by Claudia Hartley BVSc, CertVOphthal, DipECVO, FRCVS and RCVS specialist in ophthalmology.