18 Mar 2019
James Warland describes approaches to manage and treat this common yet complicated disorder in cats.

Feline hyperthyroidism management can be challenging due to variable responses, owner preferences, side effects and comorbidities, necessitating an individualised approach to each case.
This article will discuss common comorbidities in hyperthyroid cats and individualised treatments for patients.
Hyperthyroidism, or its treatment, can cause wide-ranging clinical effects, such as on the cardiovascular and renal systems, which make management challenging. Geriatric cats will frequently suffer from comorbidities, which can further complicate the management of hyperthyroidism.
Comorbidities can either be a direct consequence of the hyperthyroid state or incidental, reflecting the older age of these patients. Cardiac disease – particularly tachycardia, arrhythmia and hypertrophy – are well-recognised consequences of hyperthyroidism. Cardiac disease is seen with increasing frequency (37% versus 71% in mild versus severe hyperthyroidism) and consequence (0% versus 16% dyspnoeic), with increasing hyperthyroidism severity1.

Chronic kidney disease (CKD) is frequently recognised in cats with hyperthyroidism, but is also common within the non-hyperthyroid geriatric population; and while, to some extent, CKD and hyperthyroidism represent independent, comorbid conditions, increasing evidence exists that hyperthyroidism drives significant renal injury. The impact (or lack) of treatment of hyperthyroidism on renal disease progression is an important consideration when managing cases. A UK report1 found around 35% of cases to have hypertension and a similar number to have renal disease. Interestingly, these comorbidities were not related to the severity of the hyperthyroidism.
The value of screening for comorbidities is frequently debated. Given the expense and long hospitalisation involved in treatment, radioiodine patients are frequently screened to rule out concurrent disease before treatment. Puig et al2 found 18% (17/94) of cases presented for radioactive iodine therapy (RIT), but without overt comorbidities, had concurrent disease, directly leading to altered clinical management (not pursuing RIT).
The most commonly identified conditions were chronic enteropathy (4/17) and alimentary lymphoma (5/17). In contrast, a study evaluating abdominal ultrasound found while 36% (193/534) had abnormal findings, only 2.2% (12/534) were not treated as a result; neoplasia was infrequently found (2.4%) and was not always a barrier to RIT.
The author takes a practical view that screening for concurrent disease should always be considered, but with greater emphasis placed on patients with concerning clinical signs or physical examination findings and on candidates for treatment that is invasive, expensive or likely to impact on quality of life (for example, thyroidectomy and RIT). As a minimum, all cats diagnosed with hyperthyroidism should have a full physical examination, blood pressure assessment, haematology, biochemistry and urinalysis (ideally including culture and protein:creatinine ratio). Further imaging should be performed on a case-by-case basis.
While the prevalence of renal insufficiency appears to be similar between hyperthyroid cats and age-matched controls, it is unclear to what extent hyperthyroidism is damaging to kidney function versus being concurrent diseases in ageing cats. Renal function in feline hyperthyroidism is reviewed in detail elsewhere3. Urinary markers of tubular injury, such as N-acetyl-ß-D-glucosaminidase, are elevated in hyperthyroidism and decrease after treatment, suggesting a deleterious effect of the hyperthyroid state on the kidneys4.
Proteinuria is a common finding in hyperthyroid cats, although the mechanism is not well understood. Proteinuria is recognised to be a risk factor for the development and progression of CKD.
Hyperthyroidism activates the renin-angiotensin-aldosterone system, increasing cardiac output and blood volume, while decreasing vascular resistance. Renal blood flow and glomerular filtration rate (GFR) are consequently increased. This can decrease serum creatinine to normal concentrations, said to be “masking” CKD. Decreased muscle mass also contributes to this effect, given associated reductions in creatinine. Around 25% of well-controlled euthyroid cats will develop azotaemia over the eight months after initiating therapy5. Despite worsening of biochemical renal parameters, the return to euthyroidism is considered to be beneficial to renal function.
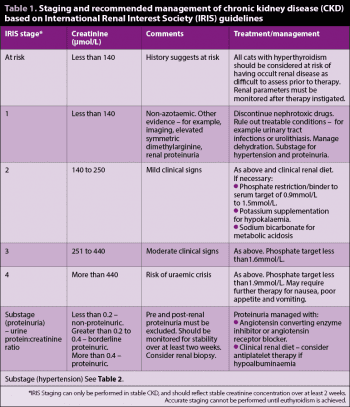
Using the International Renal Interest Society (IRIS) guidelines, most cats will show a predictable increase of up to one IRIS stage after hyperthyroidism treatment, which then remains stable7,8. Therefore, the concern regarding the development of overt CKD after treatment has likely been overstated. Given the detrimental effect of hyperthyroidism on renal health, cats with CKD should always have their hyperthyroidism managed.
Iatrogenic hypothyroidism has a negative impact on survival in cats that develop azotaemia, although not in cats without renal insufficiency6. Restoration of euthyroidism in cats with iatrogenic hypothyroidism is associated with an improvement in renal function9, and a study found azotaemic, hypothyroid cats supplemented with levothyroxine lived significantly longer than those left untreated9. Taken together, it is clear iatrogenic hypothyroidism is detrimental in cats with CKD, and clinicians should be alert to the development of this complication – particularly where renal disease is present.
Significant research has focused on predicting which cats will show renal insufficiency after treatment, but without reliable markers being identified. Cats with higher pre-treatment creatinine are at higher risk of this increasing to the point of being classified as azotaemia5. Neither proteinuria, nor low urine specific gravity (USG), are convincingly associated with the development of azotaemia after treatment in several studies5,10, although low USG was associated with post-treatment azotaemia in one publication11.

Elevated (greater than 14µg/dL) pre-treatment symmetric dimethylarginine (SDMA) does predict post-treatment azotemia11, although if only mild SDMA elevations are present, azotemia is not inevitable12. However, most cats (67%) that develop azotemia have a normal SDMA at diagnosis11.
The author recommends screening cats after diagnosis with a full urinalysis, including for proteinuria and bacteriuria, to provide a full clinical picture, despite the difficulty in predicting azotaemia with this information. Although SDMA may not change the clinical management, it may be valuable to alerting owners to the presence of CKD before undertaking hyperthyroidism treatment. Practically, in most cases, the only way to reliably identify occult renal disease is to treat the hyperthyroidism.
The hyperthyroid state is considered to be deleterious to renal health – and, therefore, previous recommendations of not treating or “under-treating” hyperthyroidism in cats with renal disease, with the intention of increasing GFR, are not recommended. Renal parameters should always be monitored alongside T4 when assessing response to treatment, and should be regularly checked for at least six months after stabilising the patient.
The author would recommend renal disease is classified and treated according to the IRIS guidelines (www.iris-kidney.com), with staging of CKD performed once stability has been achieved. The treatment recommendations for renal disease are outlined in Table 1.
The cause of hypertension in hyperthyroid cats is unclear and likely to be multifactorial. The consequences of high blood pressure on “target organs” can be devastating, including retinal haemorrhage/detachment (and blindness), renal injury, cardiac hypertrophy and brain injury. Hypertension in hyperthyroidism is associated with higher mortality5 and, while definitive evidence that treatment improves survival is lacking, management is warranted, given the potential consequences.
Elevated blood pressure is reported in 10% to 25% of cats at diagnosis13, with a further 25% developing hypertension after treatment14. Cats must be monitored for hypertension both before and after hyperthyroidism treatment. While CKD is a risk factor for hypertension, 61% of the cats that developed hypertension after treatment did not develop concurrent azotaemia. Physical examination of cats at risk of hypertension should include fundoscopy.
Guidelines for the management of hypertension in cats have been published13, including practical guidance on achieving accurate measurements, which are notoriously susceptible to stress-induced elevation. Ideally, cats should have their blood pressure measured with a Doppler system, as this is the most accurate and well-validated system. Systolic blood pressure (SBP) measurement is the most accurately measured and widely reported, so is considered more valuable than mean or diastolic pressures.
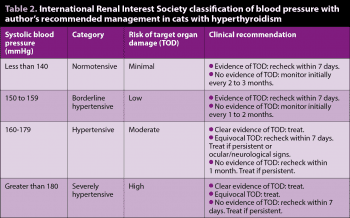
Treatment recommendations for hypertension are the same whether hyperthyroid or euthyroid. The author’s recommended management of hypertension is summarised in Table 2. Recommended initial therapy is amlodipine (0.625mg/cat/day by mouth or 1.25mg/cat/day if SBP greater than 200mm of mercury[Hg]). Most cats will respond to therapy with typical SBP reductions of 30mmHg to 70mmHg13. Dose can be adjusted up as necessary. Up to 2.5mg/cat/day can be given, although the author would usually add a second agent if inadequate control is achieved with 1.25mg/kg.
Angiotensin-converting enzyme (ACE) inhibitors (for example, benazepril 0.5mg/kg to 1mg/kg every 24 hours by mouth) or angiotensin receptor blockers (for example, telmisartan 1mg/kg every 24 hours by mouth) can be used in addition to amlodipine. Care must be taken to avoid hypotension. During stabilisation, blood pressure should be checked at least weekly. Once stable, blood pressure should be monitored every three to six months. Target SBP should be 110mmHg to 150mmHg.
Some cases with severe hypertension and target organ damage may require hospitalisation and emergency therapy to bring down SBP within hours; initial therapy can be with amlodipine as aforementioned, but parenteral treatment (for example, hydralazine) should be considered.
The importance of rechecking blood pressure after treatment of hyperthyroidism cannot be overstated. Many cats will be normotensive at diagnosis and develop hypertension after treatment – this must be recognised and treatment to ensure optimum outcomes are achieved. Alternative causes of hypertension, such as primary hyperaldosteronism, should also be considered.
Cardiomyopathy with ventricular hypertrophy, arrhythmia and thromboembolic disease is seen with cats with hyperthyroidism. Overt congestive heart failure appears to be uncommon, but likely increases with severity and chronicity of hyperthyroidism. Asymptomatic cases rarely require additional therapy (beyond treatment of hyperthyroidism), although consideration should be given to antiplatelet therapy if risk factors for thromboembolism are identified (for example, left atrial spontaneous echogenic contrast).
Interpretation of cardiac investigations, including echocardiography and N-terminal prohormone of brain natriuretic peptide measurement, can be challenging, given significant overlap between primary cardiac disease and changes secondary to hyperthyroidism15. Treatment for congestive heart failure thought to be secondary to hyperthyroidism should be as for primary cardiac disease. When assessing cardiac disease in cats with hyperthyroidism, the focus should be on whether congestive cardiac failure is present and requiring treatment, as a definitive cardiac diagnosis is unlikely to be achievable until they are euthyroid and stable for several months.
Echocardiographic changes due to hyperthyroidism are usually reversible during the months after euthyroidism is achieved15. The presence of cardiac disease or failure should not affect the treatment of hyperthyroidism per se, but may affect the suitability of the patient for certain treatments, such as surgery (and general anaesthesia).
Treatment options should be tailored to individual patients. Most cases of hyperthyroidism are due to adenomatous hyperplasia of the thyroid gland(s) and can be broadly treated with dietary, medical (antithyroid medication or radioactive iodine) or surgical management.
Uncommonly, thyroid carcinoma is reported, which is likely to be more appropriately treated with surgery or high-dose radioiodine. Thyroid cysts (benign or malignant) can be treated with surgery, RIT or a combination of these16.
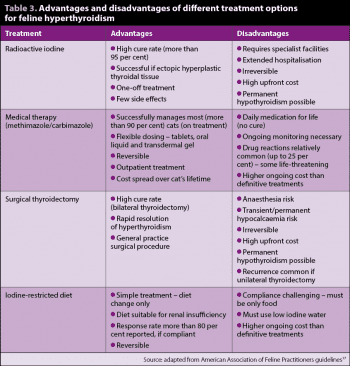
It is increasingly common to encounter cats with very mild or no clinical signs of hyperthyroidism, due to owner (mild signs) or clinician diligence (thyroid nodule), or via wellness blood screening. These cases can seem particularly challenging as performing surgery, RIT or starting life-long medication can feel overzealous. Assuming the diagnosis has been confirmed – ideally through repeat T4 measurement at an external laboratory – the author would recommend these cases are treated for their hyperthyroidism. The hyperthyroid state is progressive, and deleterious to renal and cardiovascular health, so treatment should be undertaken; any of the treatments recommended for symptomatic cases are appropriate. Treatment options for hyperthyroidism are summarised in (Table 3)17.
Treatment with radioactive iodine (i-131) is considered by most specialists to be the gold standard approach to hyperthyroidism. Cure rates are more than 95%, although controversy exists as to optimum dosing for cats. Survival times are typically excellent, with medians reported of three to four years18,19. The key advantages of RIT include high permanent cure rates, with no further treatment needed; treatment of extrathyroidal tissue and few side effects. The disadvantages are periods of hospitalisation (one to three weeks depending on centre), high up-front cost and lack of reversibility. Owners must be adequately counselled about the possibility of iatrogenic hypothyroidism, which may require supplementation – particularly as many owners choose RIT for a “medication-free” future.
Two licensed oral antithyroid medications are available for cats – methimazole/thiamazole and its prodrug, carbimazole – which act to block the biosynthesis of thyroid hormones. These effectively control hyperthyroidism, with most becoming euthyroid in two to three weeks. Initiation of medical treatment is typically more “gentle” than historic recommendations, with the aim of avoiding hypothyroidism. Typical starting doses are 1.25mg to 2.5mg/cat twice daily for methimazole and 10mg/cat once daily for carbimazole. Dose adjustments for methimazole should be 1.25mg to 2.5mg/cat/day after assessment at three weeks; the aim should be to maintain euthyroid status, without over-suppressing or under-suppressing thyroid function.
Relatively common:
More rare:
Transdermal administration of methimazole is becoming increasingly popular and may be a more convenient route of administration for many owners. It is typically applied once or twice daily to the inner pinnae. It appears to be efficacious with similar doses to oral treatment, although it appears more variability in compliance and thyroid concentrations may exist20. Other reports suggest success rates are lower and slower than with oral treatment (67% versus 82% euthyroid at four weeks)21.
It should be noted transdermal methimazole is not licensed and clinicians must be able to justify its use over oral therapy to use it under the cascade. Owners should be aware of potentially increased exposure to methimazole, which is a known teratogen; gloves should always be worn when applying the product and it should not be handled by pregnant or breastfeeding women. For the same reason, tablets should not be crushed or split. Environmental exposure of methimazole is likely to be higher with the transdermal formulation. Most side effects can be seen both with oral or transdermal methimazole or oral carbimazole, but gastrointestinal side effects are typically less common (less than 5%) with transdermal application (Panel 1)20.
Surgical thyroidectomy is associated with cure in more than 90% of cases if performed by an experienced surgeon23. Euthyroidism is usually achieved quickly, within 24 to 48 hours of surgery. If unilateral surgery is performed then around 30% to 60% of cases may recur in the contralateral gland17.
The main cause of recurrence after bilateral thyroidectomy is the presence of ectopic hyperplastic thyroidal tissue, which is reported in up to 9% of cats23. The main risks of surgery are the anaesthetic and postoperative hypocalcaemia, which occurs either transiently or permanently due to damage to the parathyroid glands. Reported incidence is variable (6% to 82%) and more common with bilateral surgery. Postoperative hypocalcaemia is usually transient, but potentially life-threatening, and cats must be adequately monitored after surgery. Cats that develop hypocalcaemia should receive vitamin D supplementation (for example, alfacalcidol) and may require emergency IV calcium borogluconate if symptomatic. Thyroidectomy may cause hypothyroidism, which can be transient (unilateral surgery) or permanent (bilateral surgery).
A commercial iodine-restricted diet is available for the management of hyperthyroidism. The key advantage of this approach is it requires minimal intervention for the patient. Dietary management appears to bring total T4 (TT4) concentration within reference intervals in the majority of cats – particularly those mildly affected, although clinical improvement is inconsistent24, 25.
Dietary management requires strict compliance, including consideration of cats eating treats, water supply (must be low iodine), other medications or finding food elsewhere, so is unlikely to be appropriate for many patients. Multi-cat households may struggle to achieve compliance, although a study has suggested normal adult cats can be safely fed the diet if it is necessary to change the diet of all cats26. Palatability appears to be generally good, with 88% accepting the change24. Interestingly, several studies have shown decreases in creatinine on dietary management, so it appears to be safe in renal insufficiency24,25. The manufacturers have designed the diet to be appropriate as a “renal diet”. In the author’s experience, dietary management is most appropriate in relatively mildly affected, indoor cats, where compliance can be ensured.
It is vital to monitor cats physical condition after treatment, particularly considering possible treatment side effects and evidence of treatment efficacy (for example, weight gain and appetite). TT4 should be checked around three to four weeks after definitive treatment or dose adjustments, and then rechecked at three and six months. When using medical therapy, TT4 should be targeted to the lower half of the reference interval. If TT4 is below the reference interval, medical treatment dosing should be reduced. Low TT4 is commonly seen one month after RIT and this often normalises over the subsequent months. This temporary, subclinical hypothyroidism is unlikely to be clinically significant unless renal disease is present or clinical signs develop, in which case hypothyroidism should be treated with supplementation. Thyroid stimulating hormone (TSH) can be used alongside T4 to diagnose hypothyroidism. Iatrogenic hypothyroidism is discussed further later.
Serum biochemistry and urinalysis should be checked to monitor renal function. In cats receiving medical therapy with methimazole/carbimazole, haematology should be checked alongside biochemistry to rule out blood dyscrasias and hepatopathy.
Blood pressure should be monitored both before and after treatment for hyperthyroidism. Many cats will be normotensive at diagnosis and develop hypertension subsequently.
Permanent or temporary iatrogenic hypothyroidism can develop subsequent to radioiodine therapy or surgical thyroidectomy. Overzealous medical management can lead to hypothyroidism, reversible with reduction/cessation of therapy. Temporary hypothyroidism is common following radioiodine, with low TT4 measured after one month, and this usually requires no further therapy as the residual function will improve over the subsequent three to six months. Reported prevalence of permanent iatrogenic hypothyroidism depends on radioiodine dose and the criteria used for diagnosis, meaning it is hugely variable, from 5% to 83%8. Clinical signs of hyperthyroidism are summarised in Panel 2.
Clinicians should aim to clinically control the cat’s signs, with the TT4 in the lower half of the reference interval. TT4 levels below the reference interval should generally be avoided. The diagnosis of hypothyroidism can be challenging as clinical signs, such as weight gain, can overlap with normal recovery from hyperthyroidism; and many euthyroid cats have concurrent disease, leading to euthyroid sick, low T4 levels. TSH has been shown to be more sensitive and specific for the detection of hypothyroidism, and measurement of TSH should be performed where hypothyroidism is suspected27. Some cats will have T4 concentrations within the reference range, so hypothyroidism should not be discounted with a normal T4. In cats that show clinical signs or are azotaemic, the author would measure TSH if the T4 is in the lower 25% of the reference interval. Unless early post-treatment, the diagnosis can be confirmed with a low-normal or low T4/free T4 and concurrent high TSH.
The author would not attempt to diagnose iatrogenic hypothyroidism in a well, non-azotaemic cat until at least three to six months after definitive therapy (radioiodine or thyroidectomy), given the high prevalence of transiently low T4 of little clinical significance, and expectation TSH will rise after treatment. The diagnosis should be pursued sooner in azotaemic cats as iatrogenic hypothyroidism shortens survival times and correction is important to improve renal function. In these cats, temporary treatment while transient hypothyroidism resolves would be indicated.
Treatment of iatrogenic hypothyroidism is to reduce the methimazole/carbimazole dose, if the cat is receiving it, or to supplement with levothyroxine (10μg/kg/day to 20μg/kg/day), which can be adjusted to maintain normal T4 and TSH. If iatrogenic hypothyroidism is permanent then treatment must be continued for life (Panel 2).
The identification and management of comorbidities are vital to ensuring optimal management of hyperthyroidism.
Renal disease and hypertension are frequently encountered, but clinicians must remember to monitor for these after treatment of hyperthyroidism, as they may only be revealed after treatment is instigated. Importantly, renal disease should not be seen as a reason to under-treat the thyroid disease, as this has further detrimental effects on renal function. Iatrogenic hypothyroidism must be avoided in these cases.
This article’s take-home messages are: