14 Sept 2021
Miguel Martinez LdoVet, CertVA, DipECVAA, MRCVS and Victoria Twist RVN discuss how relevant aspects of this human health care concept can be adapted for use in a veterinary setting.

Providing a cat-friendly environment with the use of cat hides and synthetic pheromones.
Enhanced recovery after surgery (ERAS) is a relatively new concept applied in the human medical profession. It was first recognised by Danish professor of surgery, Henrik Kehlet, in the 1980s.
ERAS takes an evidence‑based, multidisciplinary approach to perioperative care with the main aim of accelerating the recovery period and minimising complications. One documented example in humans is in those undergoing gastrointestinal (GI) surgery, where it was found the use of ERAS protocols resulted in reduced hospital stays and lower complication rates (Pędziwiatr et al, 2018).
ERAS protocols divide the perioperative period into three distinct components: preoperative, intraoperative and postoperative. Within each area, several factors can be addressed to achieve ERAS.
This article will look at how the relevant aspects can be adapted to veterinary patients.
Minimising the stress response has been identified as a key factor in ERAS.
The immediate physiological stress response leads to activation of the sympathetic nervous system (SNS). This can be in response to varying noxious stimuli, such as change in environment, surgical manipulation, starvation or any event that disrupts homeostasis.
The physiological changes brought about by the stress response prepare the body for the fight or flight response, with the aim of maintaining homeostasis. However, when prolonged, it leads to stimulation of the hypothalamic‑pituitary‑adrenal axis characterised by the release of cortisol and catecholamines.
The increased sympathetic stimulation also results in the suppression of insulin, causing hyperglycaemia. The effects can become detrimental to the patient, leading to impaired immune function and delayed wound healing.
In our patients, the stress response can be initiated even before arriving at the practice – for example, as soon as the cat is placed in its carrier, during travel or when the dog enters the veterinary clinic car park. This is known as psychogenic stress and can occur as a result of classical conditioning as the patient begins to associate the carrier or the veterinary practice with a painful or stressful experience.
It is possible to facilitate a positive patient experience in the veterinary clinic by managing environmental stressors, early socialisation and training using positive reinforcement to modify behaviour. Environmental stressors can be mitigated by the provision of a quiet environment, using synthetic pheromones, housing different species separately and avoiding patient‑to‑patient interactions.
Understanding animal behaviour is essential so that appropriate techniques for handling and restraint can be used to avoid exacerbation of stress, and to safeguard the welfare of both patients and staff. Offering treats to patients and visiting the practice for a fuss from staff is a form of positive reinforcement, and will aid in behaviour modification. Regular puppy checks play a crucial role in early socialisation and this should be reinforced to owners to ensure participation.
For known fractious or anxious patients, pharmacological management can be considered. A study by van Haaftan et al (2017) suggested administering a single dose of gabapentin in cats can help to reduce stress and aggression prior to travel and veterinary examination. In dogs, a study by Gilbert‑Gregory et al (2016) found the administration of trazodone reduced stress‑related signs and behaviours such as lip licking, panting and whining in hospitalised dogs.
To discuss in depth the minimisation of stress in the veterinary clinic is beyond the scope of this article, but as advocates for our patients we need to think about measures we can take to limit this – and those aforementioned are not an exhaustive list. Minimising stress for patients in the veterinary hospital (Lloyd, 2017) offers practical recommendations on how to achieve a “fear free” veterinary practice.
Maintaining normothermia is crucial in ERAS. Hypothermia has been described as a common yet preventable complication of anaesthesia. A study by Pottie et al (2007) found a reduced core body temperature can negatively affect the duration and quality of the recovery.
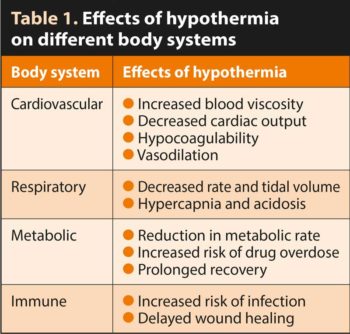
Hypothermia can have a negative impact on different body systems, as summarised in Table 1.
From the time of premedication to induction of anaesthesia, patients lose on average 0.5°C to 1°C (Armstrong et al, 2005). Pre‑warming patients can offset this initial loss. Read et al (2014) suggested the most effective method of pre-warming is using a forced warm air blanket for 30 minutes for cats and small dogs, and 45 minutes for larger dogs.
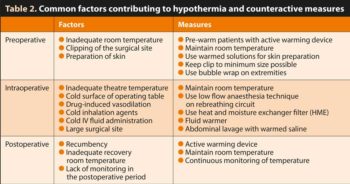
Table 2 identifies common factors contributing to hypothermia and how to counteract them.
Nutrition is an integral component of ERAS, from preoperative fasting to early enteral nutrition in the recovery period.
For an ERAS protocol to be successful, the nutritional status of the patient should be addressed before the day of surgery to optimise body condition and nutritional intake. This can be achieved in consult by the veterinary surgeon or veterinary nurse taking a clinical history from the owners, to ensure the patient is receiving a balanced diet and has a good body condition score. This is important as both extremes of patient weight have been identified as increased risks of anaesthetic death (Brodbelt et al, 2008).
Routine fasting of patients prior to anaesthesia is carried out to limit the occurrence of regurgitation and aspiration of gastric contents. However, prolonged fasting can lead to hypoglycaemia.
The widely accepted protocol of “nil by mouth after midnight” was taken from the human medical profession, and only recently has more research been carried out in dogs and cats. As a result, conflicting evidence exists on what the optimum fasting duration should be.
Viskjer and Sjöström (2017) found patients given a light meal three hours prior to anaesthesia had a significantly higher occurrence of reflux and regurgitation compared to patients that were fasted overnight. However, Savvas et al (2016) found a light meal three hours prior to anaesthesia reduced the risk of reflux compared to patients given the same volume and type of food 10 hours before anaesthesia. The results of these studies directly contradict one another, suggesting more research is needed within this area.
In the authors’ opinion, similar fasting protocols can produce different outcomes among practices due to other contributing factors, such as the type of food given and the anaesthetic protocol that was used. They suggest carrying out a clinical audit within each practice to devise a centre‑specific optimal preoperative fasting policy.
As aforementioned, minimising the stress response is vital.
In humans it is well documented that the magnitude of the response is directly proportional to the severity of the operative trauma (Desborough, 2000). This is why minimally invasive surgical techniques are used whenever possible in human ERAS protocols.
The benefits include decreased postoperative pain and faster recovery times compared to standard surgical techniques.
Although the use of these techniques is expanding in the veterinary profession, clinics may be limited by the cost of equipment and the requirement of specialised training. Even minor procedures – from taking blood samples to placing IV catheters – will elicit a surgical stress response.
Additionally to the surgical technique, the placement of drains and tubes will increase the magnitude of the surgical stress response.
Locoregional anaesthesia has been identified as an inhibitor of the stress response to tissue injury by blocking the afferent input from the surgical site to the CNS. This prevents the adrenocortical and glycaemic responses to surgery.
In veterinary literature, a study by Romano et al (2015) compared the effects of regional anaesthesia versus opioid administration on adrenocortical and glycaemic responses, postoperative pain and recovery quality in dogs undergoing stifle surgery. It was found the quality of recovery of the dogs that received opiates was noticeably worse. Additionally, they had significantly increased levels of cortisol and glucose concentrations compared to the patients that received nerve blocks.
This suggests locoregional anaesthesia would be a superior choice to opiates to enhance recovery.
Placement of an oesophageal temperature probe will provide a constant reading, but if this is not available then rectal temperatures can be taken at regular intervals to identify trends.
Monitoring ensures appropriate action can be taken to prevent hypothermia, but to also avoid overheating the patient.
Methods of active warming have shown to be superior in efficacy compared to passive methods (Franklin et al, 2012). Forced warm air blankets have been identified as the most effective, but pose an increased risk of infection – so the surgical site should be secured with drapes before the unit is turned on (Henderson, 2012).
In humans, goal-directed fluid therapy (GDT) has proven a key element in the success of ERAS protocols. GDT has been described as the optimisation of cardiac output to improve oxygen delivery and tissue perfusion. The aim is to minimise the risks of fluid imbalance during surgery to include fluid overload and hypovolaemia.
In human literature, increased rates of patient morbidity and mortality with both insufficient and overzealous fluid administration have been documented.
The current recommended guidelines for dogs is 5ml/kg/hour and for cats is 3ml/kg/hour, but a topic review by Naden (2020) found no strong evidence to support this – therefore identifying a need for further clinical studies.
The continual haemodynamic assessment of our patients is vital in the recognition and appropriate treatment of fluid deficits. This should be done on an individual basis. Fluid status can be assessed through physical examination and data analysis from monitoring equipment.
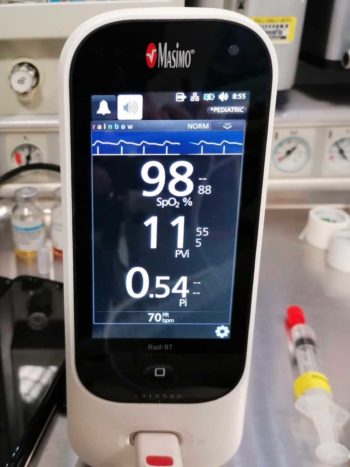
In humans, stress is placed on predicting the fluid responsiveness of a patient to determine whether a fluid challenge (fluid bolus) should be given to optimise circulation and tissue perfusion. Placement of an arterial line can provide a measurement of pulse pressure variation; however, a pulse oximeter can provide a non-invasive, more accessible option of measuring plethysmography variability index. Current literature suggests both of these parameters can be reliably used to determine the position of a patient on the Frank‑Starling curve (Sano et al, 2018).
The greater the fluctuation indicates the patient has insufficient preload, suggesting it is likely to be a fluid responder. This is particularly useful in patients that require cautious administration of IV fluid therapy, such as those with cardiac disease.
In ERAS protocols for humans, early mobilisation is encouraged to prevent risk of thromboembolic disease, muscle weakness, atelectasis and insulin resistance.
The implementation of an ERAS programme combined with mobilisation targets a reduced length of stay within hospital.
In veterinary patients, the goals of early ambulation are pain management, improved circulation to facilitate wound healing, maintaining a range of movement, and preventing infection. The ambulation goals of patients would vary greatly depending on the surgical procedure performed.
Early ambulation of veterinary patients also has several welfare benefits in the hospital environment, including the opportunity to urinate/defecate, preventing both physical discomfort from retention and psychological distress; improved hygiene, reducing the risk of infection; and reduced stress, as patients are able to express normal behaviours.

The management of postoperative pain can improve patient welfare, create a more positive patient experience and improve the quality of recovery. Bini et al (2018) carried out a comparison of two analgesic strategies after uncomplicated tibial plateau‑levelling osteotomy (TPLO) in dogs. One group received methadone every four hours and the other group had a peripheral nerve block (PNB), so was administered methadone postoperatively dependent on the pain score (PRN). Both groups had similar postoperative pain scores and neither had superior improvement in short‑term outcome. However, the group that received methadone every four hours experienced more side effects, was more likely to vomit and vocalise, and food intake was 38 per cent lower than the group that received a PNB and methadone PRN.
This suggests locoregional anaesthesia can help improve the quality of recovery compared to methadone alone, as it can provide a similar level of analgesia, but without the associated side effects of opiates.
It also highlights the importance of pain scoring in the postoperative period, as without it not only does a risk exist of providing inadequate analgesia, but more opioid‑related side effects may also occur.
Another study supporting the use of opiate‑sparing techniques was carried out by Palomba et al (2020). It compared the perioperative effects of a PNB versus a constant rate infusion of fentanyl in dogs undergoing TPLO. The dogs that received a PNB had a lower incidence of bradycardia, nociception and methadone requirement, while food intake was greater.
In both studies, patients that received a PNB were more likely to eat in the recovery period, which leads on to the next factor.
In the postoperative period, it is likely patients will be in a negative energy balance – and, therefore, a catabolic state – due to the fasting period and metabolic changes.

Early nutrition in the surgical patient is important as both the action of fasting and provision of anaesthetic agents affects GI motility and function. Fasting also increases the rate of bacterial adherence and translocation, therefore increasing the risk of sepsis.
A study by Sanderson et al (2017) found that GI motility reduces significantly after 12 to 24 hours of fasting, so it is essential to provide enteral nutrition to the patient once it has recovered from anaesthesia.
Early enteral nutrition contributes to improved GI function, decreased GI permeability and improved patient outcomes (Firth, 2013).
Patients in the recovery period may have a loss of interest in food, so highly palatable food should be offered. Palatability can be increased by warming the food. Additionally, a food that is calorie dense is ideal so the patient can obtain more calories in a smaller amount of food, especially if inappetent.
The amount of food provided should be calculated based on the patient’s resting energy requirements (RER) using the following calculation:
RER = (bodyweight [kg])0.75 × 70
Food consumption should be monitored closely to ensure adequate intake, and further measures – such as assisted feeding or appetite stimulants – can be put in place if not.
The incidence of postoperative nausea and vomiting (POVN) has not been documented in small animals, whereas in humans it is reported that 25 per cent to 30 per cent of humans experience POVN.
The provision of antiemetic prophylaxis could be considered in patients that display signs of nausea, including salivation, excessive swallowing, yawning, shivering or a depressed mentation.
Postoperative hypothermia should be addressed immediately for reasons previously mentioned. Active warming and monitoring of temperature should continue until the patient has reached a normal temperature. Patients that are non‑ambulatory due to the nature of their condition, sedation or locoregional anaesthesia may have compromised ability to thermoregulate, so it may be necessary to continue monitoring temperature even once normothermic.
ERAS protocols in human medicine are procedure‑specific and have a wealth of research behind them that continues to be developed to achieve the best possible outcomes for patients.
Although the implementation of ERAS protocols in the veterinary profession requires further research, we can begin to consider the key elements and the actions we can implement now.
Encompassing both evidence‑based veterinary medicine and a team approach, we can achieve the best outcomes for our patients.
Our focus should be on mitigating the stress response to surgery, optimising nutritional status, using opiate‑sparing analgesia techniques, and minimising the risk of hypothermia and fluid imbalance during the perioperative period.