4 May 2022
Moranne Frejlich, MRCVS, outlines the clinical signs and diagnosis of this common condition, and the treatment options available.

Keratoconjunctivitis sicca (KCS) – or “dry eye” – is a common condition of the ocular surface in dogs, resulting from a deficiency of the aqueous portion of the tear film. Although KCS can be diagnosed readily with a thorough ophthalmic examination, the diagnosis is often overlooked. This condition can be congenital or acquired, and acquired disease may be primary (immune-mediated) or secondary to systemic diseases, infection, certain drugs and iatrogenic or nerve damage.
Initially, clinical signs of KCS include blepharospasm, mucoid to mucopurulent ocular discharge, and conjunctival hyperaemia. Secondary bacterial infection may also occur. With chronicity, corneal epithelial hyperplasia, pigmentation, neovascularisation and corneal ulceration can become apparent. Ultimately, KCS can lead to extensive inflammation, pain and loss of vision.
The diagnosis of KCS is based on the presence of consistent clinical signs and measurement of decreased aqueous tear production using the Schirmer tear test. Most cases are medically managed, often with a multimodal therapeutic plan consisting of tear stimulants; tear replacements, antibiotics and anti-inflammatory agents may also be required. Alternative treatments, such as parotid duct transposition or a ciclosporin implant, may be considered for refractory or intractable cases.
Keratoconjunctivitis sicca (KCS) is a common ocular disease in dogs1,2, but is frequently misdiagnosed for simple conjunctivitis3,4. It is characterised by an aqueous tear deficiency, resulting in desiccation and progressive inflammation of the conjunctiva and cornea, which can result in ocular pain and reduced vision4-6.
The precorneal tear film (PTF) is a specialised multilaminar complex containing various enzymes, proteins, lipids and salts essential for maintaining ocular surface health and clear vision, because it is the first refractive surface of the eye.
The functions of tears are lubrication, nutrition of the cornea, flushing debris from the corneal surface, and antibacterial action4,5.
The triple-layered PTF is composed of the outer oily lipid layer, the middle aqueous fluid layer, and the innermost mucin layer7.
The lipid layer is secreted by the meibomian glands, and is thought to stabilise the tear film and reduce evaporative loss8-10.
The aqueous component of canine tears is secreted by lacrimal glands of the orbit and nictitating membrane11,12, and provides many of the metabolic needs of the avascular cornea as well as serving a lubricating function4.
The mucous layer is secreted by goblet cells present in the conjunctiva. This layer helps to provide a smooth refractive surface over the cornea, lubricates the cornea and conjunctiva, anchors the aqueous tear film to the corneal epithelium, inhibits bacterial adherence and prevents desiccation13,14.
Over the past two decades, emerging research regarding tear film dynamics suggests no distinct boundary exists between the three “classic” components of the PTF, as the lipid, aqueous and mucin layers are intricately intertwined15,16.
Lacrimal secretory disease can be divided into quantitative KCS or “dry eye syndrome”, when the aqueous component of the tear film is deficient5,6, or qualitative KCS, when the quality of the tear film is diminished, usually due to disorders of the lipid or mucin layer of the tear film5. Quantitative KCS is more common in canine patients4.
Clinical signs of KCS can vary with the severity and chronicity of the disease4. The loss of tears’ nutritive and protective functions results in a spectrum of acute and chronic corneal and conjunctival lesions6.
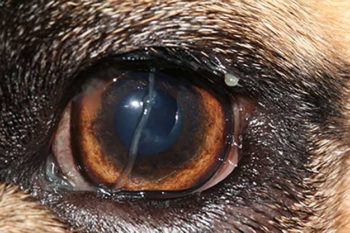
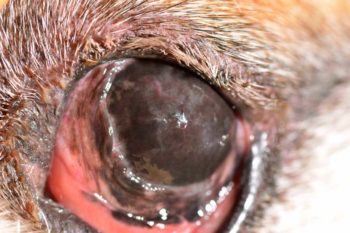
KCS typically develops gradually over a period of several weeks, with the affected eyes initially appearing red and inflamed with intermittent mucopurulent discharge (Figure 1).
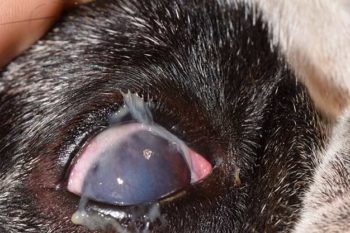
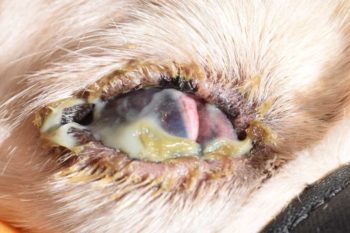
Upon increasing severity, the corneal surface appears dull, conjunctival hyperaemia worsens, and tenacious mucopurulent discharge is observed (Figures 2 and 3).
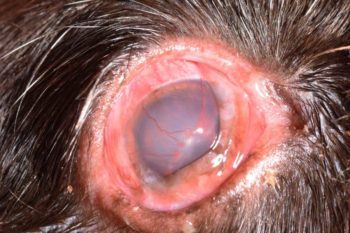
If early diagnosis and therapy are not provided, progressive keratitis characterised by extensive corneal vascularisation (Figure 4) and pigmentation (Figure 5) may develop, and corneal ulceration (Figure 6) may occur4-7.
The diagnosis of canine quantitative KCS is relatively straightforward5 and although multiple ways exist to diagnose an aqueous tear deficiency in dogs17, the Schirmer tear test (STT) remains the gold standard4 (Figures 7 and 8).
The STT is performed by placing the tear strip in the ventral conjunctival fornix, approximately midway between the medial and lateral canthus. The paper strip should not be handled excessively, as oil present on fingers may affect its absorption dynamics.
The strip should be left in place for one minute, and the measurement immediately recorded17.
During an ophthalmic examination, it should always be the first test carried out to avoid excessive reflex tearing caused by manipulation of the ocular structures, use of light and diagnostic eye drops, which may lead to a falsely increased result18.
Sedation may be used as an adjunct to manual restraint to facilitate handling and reduce anxiety associated with the ophthalmic examination.
Clinicians should always bear in mind that most of the commonly used sedatives and anaesthetics lead to a significant reduction in tear production. It is, therefore, advised to perform STT readings non-sedated and non‑anaesthetised if possible.
Readings of less than 10mm wetting in one minute confirm KCS, while readings of 11mm to 14mm are suspicious in dogs (Figure 7). Normal tear production in dogs is considered when readings measure 15mm/minute or higher4,5,7.
Before a diagnosis of KCS can be made, low readings and clinical signs of disease must be correlated, as patients can show fluctuations in STT values above and below the normal limit4,5. Quantitative tear testing should be considered as part of yearly health examinations for breeds prone to KCS19,20.
Generally, a thorough ophthalmic and physical examination pinpoints the primary cause of KCS, but occasionally blood tests or other complementary procedures are required to provide a definitive diagnosis5,21,22 (Table 1).
Causes of quantitative KCS in dogs
Quantitative KCS can be congenital or acquired, with the latter due to either ophthalmic or systemic disorders4,5.
The causes of KCS are outlined in Table 1. The most common cause of KCS is immune-mediated, and this generally
occurs in older dogs and several breed predispositions are known (Table 1). Although the majority of dogs diagnosed with KCS are of advanced age, it is unknown whether age-related lacrimal/third eyelid gland atrophy exists in dogs as a cause of KCS24,50.
Most cases of “dry eye” in dogs respond well to medical therapy. A variety of therapeutic options are available.
Therapeutic regimens are tailored to each individual and influenced by the underlying pathogenesis, disease severity and owner’s preference. Generally, treatment consists of a combination of tear stimulation and replacement agents. Topical antimicrobials, anti‑inflammatory therapy and mucolytic therapy may also be added if indicated4,5,7 (Table 2).
KCS treatment has three main objectives: restoring natural tear production, maintaining hydration and lubrication of the corneal surface, and restoring its transparency.
Due to the progressive and chronic nature of this disease, it is imperative that owners are made aware of the need for regular follow-up examinations and lifelong medication.
Due to the adequate medical management of a high percentage of KCS cases, surgical procedures to treat tear deficiencies have evolved little in recent decades. In some patients that fail to improve with medical treatment, surgery may be considered4,5,54.
Traditionally, parotid duct transposition (PDT) and permanent partial tarsorrhaphy have been the most common surgical treatments for KCS cases. These procedures provide saliva as a substitute for tears and reduce exposure of the eye, respectively4,5.
More recently, subconjunctival CsA implants have been developed for cases with low treatment compliance or tolerance55,56.
Saliva and tears have many similarities, including similar osmolarity and pH; however, the mineral contents differ significantly4.
Dogs with KCS may have xerostomia (dry mouth), and these animals are not suitable candidates for this procedure. Testing the flow of salivary fluid from the parotid duct is done easily by administering a bitter substance (for example, one drop of ophthalmic atropine solution) to the tongue and observing the salivary flow from the parotid papilla5.
Two techniques are described for this procedure, and both require magnification, fine surgical instruments and meticulous skills; therefore, specialist referral is recommended7,57. The parotid duct can be approached by either the lateral (open method) or oral (closed method) route.
In general, the lateral approach provides better exposure, reduces the risk of duct transection and significantly reduces septic contamination of the incision4,57.
Although PDT can provide comfort and moisture for severely affected dogs, perioperative, postoperative and long-
Postoperative complications of PDT may include dermatitis due to saliva overflow around the eyes, and a buildup of salivary mineral precipitates on the cornea and eyelid margins, leading to chronic ulcerations and periocular irritation.
Both the quality and the quantity of saliva transported to the eye contribute to the appearance of these adverse effects4,5,58.
Sustained-release ocular implants have been developed over the past decade to deliver therapeutic levels of drugs to the eye over a longer period of time, eliminating or minimising the effects of non‑compliance of patients and owner55,56.
The subconjunctival and episcleral spaces have been proposed as most appropriate sites for ciclosporin A delivery to the lacrimal glands. A subconjunctival or episcleral pocket is created while the animal is under general anaesthesia, the implant is placed in the pocket, and the conjunctiva is closed using a simple, interrupted pattern55.