25 Mar 2019
James Warland describes taking a logical route to diagnosing this condition, including laboratory work and imaging methods.

Figure 1. The sclera is normally the first site jaundice can be seen.
Liver disease can be challenging to accurately diagnose due to vague presenting symptoms and the lack of disease-specific diagnostic findings. However, by using a logical approach to possible liver disease, important information can be obtained from blood and imaging results, and clinical decisions about the need for biopsies and treatments can be made.
The clinical presentation of liver disease in dogs and cats is variable, owing to the number of important functions of the liver, and that variation in severity of damage to different microscopic regions will lead to different physiological effects.
Acute and chronic insults may present differently, although deterioration of chronic disease can present in an acute fashion. The liver’s large functional reserve capacity means many animals will present with minimal, vague or no clinical signs, and clinicians are often challenged to investigate incidentally found elevated liver enzymes. The lack of obvious clinical signs can make deciding how urgently to pursue sometimes invasive investigations particularly difficult.
The liver also has immunoregulatory roles.
Early clinical signs of liver disease tend to be non-specific, such as inappetence/anorexia, lethargy, weight loss, vomiting, diarrhoea, and polyuria/polydipsia (PUPD). With more severe disease, specific clinical signs, directly attributable to liver function – such as jaundice/icterus, ascites, hypoglycaemia, coagulopathy and hepatic encephalopathy – can develop.
The patient signalment and history often provide important clinical information. Some liver diseases have strong breed predispositions, such as copper storage disease in Bedlington terriers, Labrador retrievers, Dobermanns and Dalmatians, among others. Several breeds are recognised to suffer from idiopathic chronic hepatitis, including English springer spaniels, Labrador retrievers and cocker spaniels.
Congenital portosystemic shunts (CPSS) are also recognised to have breed predispositions, with extrahepatic CPSS most common in small breed dogs (such as Maltese terriers, Yorkshire terriers, pugs and miniature schnauzers) and intrahepatic CPSS seen in larger breeds (including Irish wolfhounds and golden retrievers). Scottish terriers are predisposed to a breed-related, progressive vacuolar hepatopathy.
The following does not represent an exhaustive list.
Environmental
Food supplements
Medications
Chemicals
* Modified from Cocker S and Richter K (2016). Diagnostic evaluation of the liver. In Ettinger SJ, Feldman EC and Côté E (eds), Textbook of Veterinary Internal Medicine (8th edn), Elsevier Saunders, St Louis: 1,611-1,620.
Given the importance of toxic and infectious causes of hepatopathies, it is important to obtain a detailed history, including diet, vaccination status, travel history, as well as medications, including nutraceuticals or supplements, which may not have been prescribed (Panel 1). Feline hepatic lipidosis is most commonly seen in overweight, male cats with a recent history of weight loss and anorexia.
Jaundice (icterus) is due to excessive plasma bilirubin. Once increased red cell breakdown (for example, haemolytic anaemia) is excluded via complete blood count, this indicates the jaundice is due to hepatic cholestasis or post-hepatic biliary obstruction. Typically, clinical jaundice is not visible until bilirubin concentrations exceed 40µmol/L (Figures 1 and 2).
Ascites is due to fluid accumulation within the abdominal cavity. In liver disease, ascites is most commonly due to portal hypertension (PH), with a component often also due to decreased vascular oncotic pressure owing to hypoalbuminaemia. As well as alterations to the blood flow within the liver, often due to severe fibrosis or cirrhosis, PH can be due to prehepatic obstruction (for example, compression or thrombosis of the portal vein) or posthepatic, due to obstruction of the caudal vena cava or right atrium (Figure 3).
Hepatic encephalopathy (HE) is due to brain dysfunction, secondary to circulating toxins inadequately broken down by the liver. The pathogenesis of HE is complex due to many neurotoxins, with ammonia representing the most important. Dogs appear to be more sensitive to HE than cats and present more commonly with these signs. Although HE can occur acutely, due to fulminant hepatic failure, most cases are due to chronic HE, owing to shunting of portal blood to the systemic circulation. Portosystemic shunting can occur due to a congenital aberrant vessel (usually single) or multiple, acquired shunts, resulting from portal hypertension.
Occasionally, HE can be due to failure of the urea cycle, resulting in ammonia build-up; in cats, this can occur in prolonged fasting due to deficiency of the essential amino acid, arginine. Clinical signs of HE initially include vague signs, such as listlessness and decreased alertness – worsening to ataxia, circling, head-pressing, ptyalism, seizures and coma in more severe cases.
A complete blood count can be helpful to investigate liver disease and help rule out other conditions, but does not show any specific changes. Haematological alterations commonly seen in liver disease include mild/moderate anaemia, which can relate to chronic disease or haemorrhage (gastrointestinal ulceration or due to coagulopathy), and is often microcytic and hypochromic due to impaired iron transport.
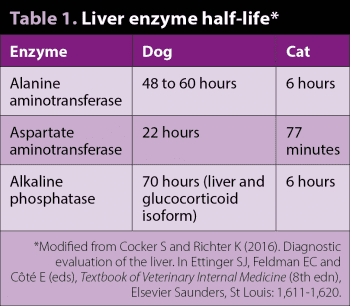
The commonly measured hepatic enzymes measured in small animal practice are alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP or ALkP) and gamma-glutamyl transferase (GGT). These are useful enzymes to measure due to relatively stable serum levels in health, meaning elevations are indicative of cell membrane injury. Importantly, different enzymatic elevations provide information on the site of hepatic injury. ALT and AST, found in the hepatocyte cytosol, are hepatocellular leakage enzymes, whereas ALP and GGT, on the hepatocyte canalicular membrane, are cholestatic enzymes, increasing due to cholestatic injury to these membranes.
Importantly, these enzymes are markers of hepatocyte injury. They do not reflect liver function and the degree of their elevation does not indicate reversibility of the disease process. Therefore, these liver enzyme elevations should not be used to provide prognostic information. However, depending on the disease process, they may be useful for monitoring patients’ clinical progress and response to treatment.
These enzymes are relatively sensitive markers of hepatic pathology, but are not specific to primary hepatic disease. The liver is reactive to systemic changes, and extrahepatic inflammatory disease, particularly of the intestines or pancreas, can lead to secondary reactive release. In dogs, typically, these are mild, but elevations of more than three to four times the reference range suggest hepatic pathology. The half-life of enzymes need to be considered when interpreting and planning follow-up blood tests (Table 1). In cats, owing to shorter enzyme half-life times, any elevation is considered significant.
It should be remembered some of these enzymes can be produced by other tissues – producing important differentials other than liver disease when elevated. ALP has bone, intestinal and renal isoenzymes, although the bone isoform is the only one that is often significantly elevated in serum (for example, growing animals, bone metastasis and osteosarcoma). GGT tends to be more liver/biliary-specific as no bone isoenzyme is present. AST is found in significant concentrations in muscle (ALT much less significantly) and erythrocytes. Creatine kinase can be valuable to distinguish muscle versus liver release of AST. Elevations in AST, without ALT increase, likely suggests extrahepatic causes (consider in vitro haemolysis).
Medications
Extrahepatic inflammation
Endocrine disease
Hypoxia
Other
* Modified from Cocker S and Richter K (2016). Diagnostic evaluation of the liver. In Ettinger SJ, Feldman EC and Côté E (eds), Textbook of Veterinary Internal Medicine (8th edn), Elsevier Saunders, St Louis: 1,611-1,620.
Enzyme induction is another important differential as increased production of these enzymes (as opposed to cellular leakage) will also cause serum elevations. It is seen particularly with glucocorticoids (exogenous or endogenous), leading to sometimes marked elevations in ALP (in dogs) and phenobarbital, which can increase ALT or ALP. No steroid-induced isoenzyme of ALP exists in cats (Panel 2).
Several biochemical markers are produced by the liver, meaning they represent useful markers of liver function. However, they are rarely specific to liver disease.
Albumin is synthesised by the liver, although significant reserve capacity means hypoalbuminaemia is not seen until approximately 70% of liver function has been lost. Hypoalbuminaemia is also commonly due to protein losing enteropathy or nephropathy. Globulins are primarily made by B-lymphocytes, meaning these are usually normal (or high) in liver disease. Concurrent hypoglobulinaemia and hypoalbuminaemia typically reflects severe gastrointestinal pathology.
Hypoglycaemia can result from liver disease – particularly in severe, acute, fulminant hepatic failure, young or small breeds, or CPSS. Glucose should always be checked in dogs showing weakness or neurological signs, and hypoglycaemia can resemble HE.
Urea (or blood urea nitrogen) is produced from ammonia – a breakdown product of protein by colonic bacteria. Urea is frequently low in cases of severe liver disease or portosystemic shunting of blood around the liver. Low blood urea nitrogen is not specific and can be due to a low protein diet, diuretics or PUPD.
Portosystemic shunts (PSS; congenital or acquired) also frequently lead to hyperammonaemia, due to failure to enter the hepatic urea cycle. Measurement of elevated ammonia provides evidence of PSS and hepatic encephalopathy; however, measurement must be performed quickly due to the labile nature of this compound. Sample handling must be very careful to obtain accurate results.
Bile acids are produced from cholesterol in the liver and excreted in bile, before being recycled through the enterohepatic circulation. The liver is very efficient at processing bile acids, meaning elevations in bile acids are typically seen in severe liver dysfunction – particularly if portosystemic shunting is occurring. Measurement of postprandial (two hours after eating) bile acids can improve the sensitivity of testing for hepatic dysfunction. Very high serum bile acid concentrations (more than 100µmol/L) are typical of PSS.
Hypocholesterolaemia can also be seen in dogs with liver disease. It is important to remember bile acids will be elevated in dogs with cholestasis and, therefore, measurement will provide no clinical information in any patient with hepatic or post-hepatic icterus.
Most clotting factors are produced by the liver and biliary stasis can also affect the absorption of fat-soluble vitamin K. In addition, liver disease can increase fibrinolysis, cause thrombocytopenia or lead to disseminated intravascular coagulation. Coagulation profile abnormalities are seen relatively commonly in dogs and cats, although unprovoked clinical bleeding appears to be rare. Clotting abnormalities must be considered when planning liver sampling, including fine needle aspirates or biopsy.
Radiography can demonstrate hepatomegaly or microhepatica, and may allow assessment of liver margins, biliary mineralisation and cholelithiasis. Loss of serosal detail can be seen in cases with ascites. However, radiographs rarely provide significant value in assessment of liver disease.
Ultrasound allows assessment of parenchymal structure and architecture, portal vasculature and the biliary tract, including intrahepatic and extrahepatic bile ducts and gall bladder. The experience and skill of the operator will significantly impact on the value of the assessment. Despite the value of ultrasound, clinicians should be aware poor correlation exists between any ultrasound parenchymal assessment and the histopathologic diagnosis1. This includes assessment of neoplasia, although large mass size and peritoneal effusion are associated with malignancy2. Ultrasound can also be used to detect free abdominal fluid (ascites).
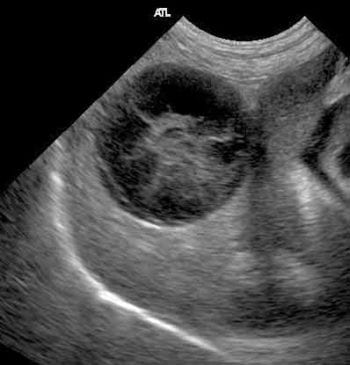
The liver should be homogenous in appearance, hypoechoic to the spleen. Ultrasound is poorly sensitive for the detection of liver disease, and many dogs will have totally normal ultrasound findings. During the examination it is important to look for focal masses; examine the bile ducts for dilation (normally less than 3mm in dogs and less than 4mm to 5mm in cats), thickening and obstruction; and the gall bladder for thickening and hyperechoic contents, including mucocoele formation (characteristic stellate, echogenic “kiwi fruit” pattern; Figure 4), cholelithiasis or sludge accumulation. The presence of sludge within the gall bladder is frequently of no clinical significance.
The portal vasculature can be assessed with ultrasound, particularly looking for portosystemic shunting. CPSS is usually seen as a single vessel and its entrance in the caudal vena cava is usually associated with turbulent flow, as seen with Doppler flow.
Dogs with CPSS will almost always have small hepatic size; and typically have portal vein:aorta ratio diameters of less than 0.65, with ratios of more than 0.8 consistently ruling out CPSS3.
Typically, multiple acquired PSS can be visualised in the left dorsal, perirenal area, as a plexus of small, tortuous, splenic to renal vessels, and occur secondary to PH. Other evidence of PH seen on ultrasound include reduced portal blood flow velocity (less than 10m/s), hepatofugal flow and enlargement of the portal vein.
Other ultrasound findings consistent with PSS include renomegaly and urolithiasis (ammonium biurate), and urinalysis to look for these crystals can also be valuable in the diagnosis of PSS.
Advanced imaging, particularly CT, is valuable in the diagnosis of some liver conditions. The author would recommend the use of CT angiography to definitively exclude PSS in suspected cases – especially in large dogs, as these cannot always be accurately visualised on ultrasound. In addition, CT can be useful for surgical planning in CPSS or mass lesions. MRI can be useful in the assessment of liver masses for prediction of malignancy and surgical planning.
For liver parenchymal disease, imaging, examination and blood findings are rarely definitive, meaning biopsies are necessary to achieve a diagnosis. Fine needle aspirates can be taken using ultrasound guidance – usually under sedation. These can be useful for defining certain diseases – particularly some neoplastic processes, such as lymphoma and hepatic lipidosis in cats. Poor correlation exists between fine needle aspirate results and histopathology4, meaning results can frequently be inaccurate or misleading. They are particularly unreliable for diffuse, chronic parenchymal diseases.
The inaccuracy of fine needle aspirates means most cases require histopathology (Panel 3). Biopsies can be obtained via a percutaneous, ultrasound-guided needle biopsy or via surgical biopsy (either laparoscopic or at laparotomy). Ideally, biopsies should be large enough to contain 3 to 12 portal triads5, although clinicians should be aware all three techniques showed only moderate correlation to necropsy-obtained samples. Multiple samples should be obtained to improve diagnostic accuracy.
The WSAVA Liver Standardization Group recommends platelet count and coagulation testing is carried out shortly before any biopsy technique is performed, with fibrinogen the most critical indicator; it does not recommend biopsy if fibrinogen is less than 50% of the lower reference interval6. Despite this recommendation, little evidence exists that risk of haemorrhage is related to coagulation parameters in liver biopsies7,8. The author would worry about biopsy in animals with severe thrombocytopenia, hypofibrinogenemia or clinical evidence of coagulopathy.
Fresh frozen plasma and red cells (or whole blood) should be available for emergency transfusion if needed. Bleeding may occur in patients with normal coagulation parameters. Prophylactic administration may be considered in cases at highest risk, and administration of vitamin K can also be considered – particularly in cats.
The author typically recommends laparoscopic surgical biopsies in the majority of cases. Surgical techniques have the advantage of producing larger biopsies, and allow visualisation of the liver to enable targeted sampling, inspection of other abdominal organs, and monitoring for haemorrhage. Laparoscopy is typically less invasive, but requires specialist equipment and experience. Laparotomy can be performed by most vets, but is more invasive; it is particularly recommended if a surgical intervention is anticipated, such as in biliary tract obstruction, to remove a mass lesion, or to allow biopsy of other organs, such as lymph nodes, pancreas or intestine.
Cats frequently suffer from concurrent inflammatory enteropathy, hepatopathy and pancreatitis (triaditis), and sampling of multiple organs may be valuable, depending on the clinical presentation.
Percutaneous biopsies have the advantage of being less invasive and expensive, but owing to the small size, may be non-diagnostic or inaccurate. In addition, particularly in cats, percutaneous biopsies have been associated with major bleeding7 and automated devices have high rates of death due to suspected vasovagal reactions9. Automated percutaneous biopsy needles should not be used in cats. The technique requires experience and is difficult to perform in small livers or in the presence of ascites. Additionally, monitoring for post-procedure haemorrhage is difficult.
In addition to taking several biopsies for histopathology, samples can be taken for quantification of copper/heavy metals and aerobic/anaerobic culture. In the majority of cases, the author would concurrently obtain a bile sample for cytology and bacterial culture, which can be particularly valuable for the diagnosis of bacterial cholangitis/cholangiohepatitis.
A few cases may be investigated using other diagnostic procedures. Leptospirosis is an important cause of acute hepatopathy in dogs, meaning PCR and/or serology should be considered in possible cases – particularly those that present with concurrent acute kidney injury. A case series of chronic hepatitis cases, without obvious renal involvement, have been attributed to leptospirosis, which were diagnosed using fluorescence in situ hybridisation, a technique that can be useful where bacterial infection is suspected10.
An important differential diagnosis for congenital PSS is congenital portal vein hypoplasia without portal hypertension (also known as microvascular dysplasia). Typically, small breed dogs usually present without clinical signs, but may have markedly elevated bile acids, and other features of PSS. A definitive diagnosis requires ruling out macroscopic shunting, best performed with CT, and histopathology. Alternatively, measurement of serum protein C activity can be helpful, as levels less than 70% are seen in the majority of cases of PSS (88%), but are rare in portal vein hypoplasia (5%)11.