2 Aug 2017
Albane Fauron and Karen Perry, in the second of a three-part article, discuss how to spot this condition in dogs and methods of examination.

Canine cranial cruciate ligament (CrCL) disease can affect a variety of breeds, although an increased incidence in certain breeds has consistently been reported. A bimodal age distribution exists, with larger dogs seemingly more likely to develop CrCL at a younger age compared to smaller dogs. Because CrCL rupture results from a degenerative process in the majority of cases, both stifles are commonly affected. Concomitant medical meniscal injuries are frequent and typically painful.
Clinical signs may vary from a subtle lameness that worsens with exercise to a toe touching lameness depending on the chronicity and extent of ligamentous damage and presence/absence of meniscal damage. A presumptive diagnosis can usually be made based on history and orthopaedic examination findings; however, radiographs may be useful to rule out other potential contributing factors.
In the first part of this article (VT47.24), the authors looked at the pathogenesis of cranial cruciate ligament (CrCL) disease. This part will discuss the typical presentation of dogs suffering with this common condition, including the signalment and clinical signs. It will also review the route to a definitive diagnosis in cases of CrCL disease.
A study of 1.25 million dogs in the US over a 40-year period identified the five breeds most commonly affected by CrCL pathology to be the Newfoundland, Rottweiler, Labrador retriever, bulldog and boxer (Witsberger et al, 2008).
Several studies have confirmed increased prevalence in these breeds, as well as reporting predisposition in other breeds, including the West Highland white terrier, Yorkshire terrier, golden retriever, Staffordshire bull terrier, Neapolitan mastiff, akita, St Bernard, mastiff, Chesapeake Bay retriever and American Staffordshire terrier (Whitehair et al, 1993; Duval et al, 1999; Adams et al, 2011; Guthrie et al, 2012).
Conversely, greyhounds and dachshunds have consistently been reported at low risk for the disease (Whitehair et al, 1993; Duval et al, 1999), while a study reported, for the first time, cocker spaniel as a breed with reduced odds of diagnosis (Taylor-Brown et al, 2015).
Vasseur et al (1985) demonstrated a consistent and progressive decrease in the mechanical properties of the CrCL in dogs more than five years old, as well as an uneven relationship between age and the severity of these degenerative changes depending on bodyweight. Indeed, the CrCL of dogs weighing below 15kg generally had less pronounced degenerative changes (such as loss of fibroblasts, chondroid metaplasia of the surviving fibroblasts and alteration to collagen fibres) than those of dogs weighing more than 15kg.
Several studies have confirmed these histopathological findings and reported a bimodal age distribution in dogs suffering from CrCL, with smaller dogs tending to be affected later in life than larger dogs (Bennet et al, 1988; Whitehair et al, 1993; Duval et al, 1998). Additionally, it has been reported dogs that only develop unilateral CrCL rupture present significantly older than dogs that develop bilateral disease (median age 7 and 4.5 years, respectively; Cabrera et al, 2008).
A study confirmed this finding, with breeds such as Rottweiler being at high risk of developing bilateral CrCL disease and presenting at a significantly younger age (median age 2.7 years; Guthrie et al, 2012).
Neutered dogs, particularly females, have been found to be at an increased risk of CrCL rupture compared to their sexually intact counterparts (Whitehair et al, 1993). In one study, neutered female dogs had 2.1 times the odds of being diagnosed with CrCL compared with entire females (Taylor-Brown et al, 2015). The possible underlying causes for this finding were discussed in part one.
A retrospective study investigating the effect of signalment on the presentation of dogs suffering from CrCL pathology found entire dogs were significantly younger than neutered dogs at presentation and, additionally, entire females presented significantly younger than neutered females, entire males and neutered males; describing, for the first time, a possible effect of gender on the age of presentation (Guthrie et al, 2012).
CrCL pathology commonly affects both stifle joints, with 14.6% to 38% of dogs reported to be bilaterally affected at the time of presentation (Guthrie et al, 2012; Fitzpatrick and Solano, 2010).
Incidence of true bilateral CrCL rupture may vary with breed, with one study reporting an incidence rate of 50% in Rottweiler and boxer breeds, while a study focusing exclusively on the Labrador retriever found only 10.2% of 94 dogs initially presented with bilateral CrCL rupture (Guthrie et al, 2012; Buote et al, 2009). Cabrera et al (2008) reported up to 61% of dogs that initially presented with unilateral CrCL pathology went on to develop contralateral CrCL rupture within 32 weeks, so this is something owners must be informed of when making decisions regarding treatment. In Labrador retrievers, contralateral deficiency was predicted to occur within 25 weeks in 48% of dogs (Buote et al, 2009).
Moreover, the likelihood of contralateral CrCL rupture increases with the presence of radiographic osteoarthritic changes; it has been shown 59% of dogs with bilateral OA went on to rupture the contralateral CrCL and the global OA score of the contralateral stifle joint was reported as a risk factor for subsequent CrCL rupture within the next six months (Doverspike et al, 1993; de Bruin et al, 2007; Grierson et al, 2011).
Several studies have showed an increase in bilateral CrCL rupture in young, large breed dogs, as well as an increased risk of contralateral CrCL rupture in these dogs after initial repair (Bennet et al, 1988; Pond and Campbell, 1972; Moore and Read, 1995).

Depending on the severity of ligament disruption, two broad clinical pictures are associated with CrCL pathology. Dogs can present with either a chronic pelvic limb lameness pronounced initially, improved over time, but never completely resolved, or with an acute non-weightbearing lameness.
The CrCL is composed of two functional parts: a small craniomedial (CrM) band and a larger caudolateral (CdL) band. The term “full tear” implies that both the CrM and CdL bands have ruptured, while “partial tear” is used to indicate only one of the two bands had ruptured at the time of diagnosis. In cases of relatively stable partial tears, a subtle weightbearing lameness that worsens after exercise might be the only historical finding (Kowaleski et al, 2012).
Conversely, the initial lameness following complete acute CrCL rupture is typically non-weightbearing. However, most animals will start to use the limb and progress to a marked to moderate weightbearing lameness within two to three weeks of the injury (Piermattei et al, 2006).
In any case of stifle instability (partial or complete), periarticular fibrotic tissue forms and progressively stabilises the joint, but not sufficiently to prevent its persistent deterioration. Hence, improvement will typically continue until secondary meniscal damage and/or osteoarthritic changes develop, and contribute to further functional decline (Piermattei et al, 2006). Dogs affected with CrCL rupture might also be reported to be sitting in an unusual position, with the affected leg projecting out to the side rather than sitting squarely, which translates a resistance to fully flex the knee when sitting (Piermattei et al, 2006; Kowaleski et al, 2012; Figure 1).
Exacerbation of lameness in a CrCL deficient stifle and/or the presence of an audible click when ambulating are both signs indicating possible concomitant meniscal injury (Case et al, 2008). Meniscal tears are common in the cranial cruciate deficient stifle and have been identified in 33.2% to 77% of dogs with CrCL pathology (Casale and McCarthy, 2009; Ralphs and Whitney, 2002; Williams et al, 1994; Fitzpatrick and Solano, 2010). When meniscal injury is associated with CrCL insufficiency, the medial meniscus is affected much more commonly than the lateral meniscus (Flo and DeYoung, 1978).
A study evaluating meniscal status in 87 dogs with CrCL disease found only the medial meniscus to be affected in 100% of cases (Bennet and May, 1991). Similarly, in a larger study, Fitzpatrick and Solano (2010) found 100% of 319 dogs with concomitant CrCL insufficiency and meniscal injury had meniscal pathology affecting the medial meniscus only.
Medial meniscus injury may result from an acute tear at the time of CrCL rupture or from more progressive damage caused by chronic instability (Flo and Young, 1978; Bennet and May, 1991; Slocum B and Slocum TD, 1993). Meniscal tears associated with CrCL deficiency are thought to occur secondary to abnormal motion of the CrCL deficient stifle. The significant discrepancy between the incidence of medial versus lateral meniscal injuries can be explained by anatomical differences that exist in their attachments to surrounding structures. Both menisci are anchored to the tibia and femur by five meniscal ligaments, and to each other by a single intermeniscal ligament.
In addition to these ligamentous attachments, the medial meniscus is also firmly attached to the tibia via both the joint capsule and medial collateral ligament (Flo and Young, 1978). Therefore, in the CrCL deficient stifle the caudal horn of the medial meniscus – which acts as a wedge between the femoral and tibial condyles – may become crushed during tibial cranial translation and the caudal horn may tear secondary to this, resulting in shear stress (Flo and Young, 1978; Pozzi et al, 2006).
The lateral meniscus, in contrast, is more mobile, allowing it to avoid this “wedge effect”; during drawer movement, the lateral meniscus is pulled backward by the femoral ligament of the lateral meniscus, so pressure of the femoral condyle is distributed within a broader area of the lateral meniscus rather than concentrated on the rim (Flo and Young, 1978).
While the meniscal body appears devoid of blood vessels and nerves, meniscal horns have a rich blood and nervous supply (O’Connor, 1976), which is why a sudden deterioration of lameness in a chronic case of CrCL rupture should raise suspicion of meniscal injury.
Additionally, the displacement of the torn meniscus during weightbearing accounts for the occasional audible or palpable click noted during ambulation or palpation. However, it is important to note while the presence of a click supports a medial meniscal injury, its absence does not rule it out (Case et al, 2008).
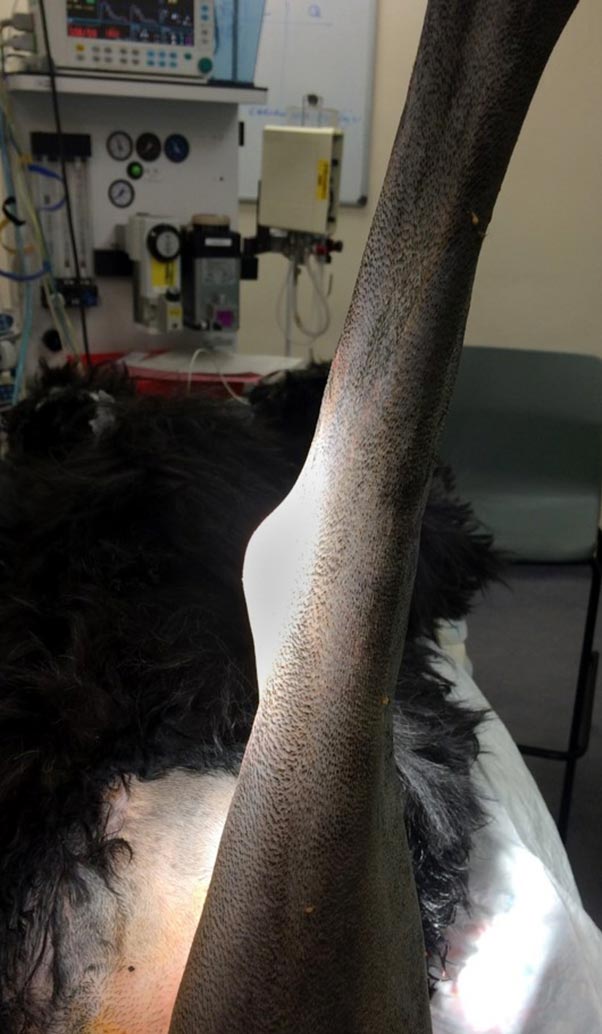
As mentioned previously, gait evaluation reveals the presence of either a subtle or more pronounced “toe touching” pelvic limb lameness, depending on the severity of ligament disruption.
Even in the presence of a history and lameness strongly suggestive of CrCL pathology, careful and thorough physical and orthopaedic examinations should be carried out to ensure possible concomitant issues are not overlooked. As with any other orthopaedic condition, the authors recommend that manipulations of areas anticipated to be painful (in this case the stifle) be performed at the end of the examination. Additionally, as mentioned in part one, concomitance of CrCL pathology and medial patellar luxation (MPL) is well recognised and, as such, the stifle should always be assessed for evidence of MPL.
The standing position allows direct comparison between limbs and facilitates detection of asymmetry. The comparative girth of the quadriceps femoris musculature should be appreciated. In chronic cases, muscle atrophy may be visually obvious or become evident on palpating both sides simultaneously and noting a discrepancy in quadriceps circumference.
The stifle joint should be assessed for synovial effusion by palpating the right and left straight patellar ligaments simultaneously. This is most easily done by standing behind the patient and running the thumb and index finger of each hand down the length of the patellar ligament (Harasen, 2002). In the normal stifle, the distinct medial and lateral edges of the tendon can easily be palpated, but become less evident in the presence of stifle effusion (Kowaleski et al, 2012).
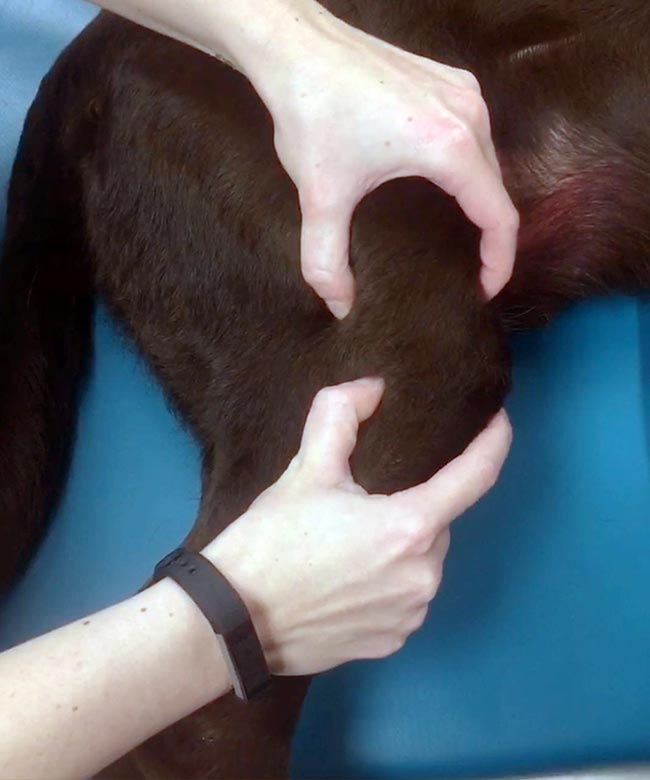
In addition to more or less pronounced muscle atrophy, chronicity also typically leads to periarticular tissues becoming thickened and fibrotic. This is often most obvious on the medial side and is commonly referred to as “medial buttress”, which can be easily appreciated by again palpating the right and left side simultaneously (Figure 2).
Definitive diagnosis of CrCL pathology relies on assessing stifle joint instability by means of the cranial drawer and tibial thrust tests. In both tests, a positive result is considered pathognomonic for CrCL deficiency. Drawer movement refers to the cranial displacement of the proximal articular surface of the tibia in relation to the distal femur.
One of the main roles of the cranial cruciate ligament is to limit cranial displacement of the tibia relative to the femur – along with preventing internal tibial rotation and stifle hyperextension (Arnoczky and Marshall, 1977). Hence, in the adult animal, the observation of any drawer motion indicates CrCL deficiency and is considered abnormal. It should, however, be noted in immature dogs some degree of instability in cranial drawer can be considered a normal finding. This is commonly referred to as “puppy drawer” and represents mild physiologic stifle joint instability. If present in a young dog, this can be differentiated from pathologic instability (such as CrCL rupture or avulsion) by the sudden endpoint of cranial drawer motion as the ligament is stretched taut. Additionally, both stifle joints will palpate similarly (Piermattei et al, 2006).
In a cranial drawer test, a direct cranial tibial translation (in respect to the femur) is created directly by applying a force to the tibia while holding the femur in place. This test is said to assess “direct” drawer movement and is typically performed in lateral recumbency.
As described by Piermattei et al (2006), a cranial drawer test is performed by placing the index finger of one hand over the patella while the thumb of the same hand is placed just caudal to the lateral fabella. The index finger of the opposite hand is placed on the cranial aspect of the tibial tuberosity, while the thumb of the same hand is placed on the caudal aspect of the fibular head (Figure 3). The operator’s fingers should be placed as close as possible to bone and securely positioned to the aforementioned landmark. Failure to do so may allow motion of soft tissue motion to be misinterpreted as stifle instability (Kowaleski et al, 2012).
Testing for direct cranial drawer may be painful and should ideally be performed under sedation, which can easily be done after radiographs have been taken and before the patient is reversed.
The tibial thrust or tibial compression test can be performed with the animal either standing or in lateral recumbency. The tibial thrust test mimics the action of the musculature of the stifle created during the stance phase of gait. In their seminal publication, Slocum and Devine were the first to identify and describe a cranially directed force within the canine stifle joint (Slocum and Devine, 1983). According to their model, ground reaction forces created during the stance phase are transmitted along the axis of the tibia and a shear force (cranial tibial thrust) is generated by the compression of the femur against the tibial plateau. Because of its cranial orientation, this femorotibial shear force leads to cranial translation of the tibia.
In a stable stifle, this translation is countered by active and passive restraints provided by the flexor muscles and CrCL and menisci respectively. In the presence of CrCL rupture, the ligament is no longer capable of counteracting the cranial tibial thrust described above, and the tibia slides forward in relation to the femur. Because in this test, drawer motion (if present) results from reenactment of forces transmitted through the stifle joint and not from direct manipulation of the tibia and femur, it is termed “indirect drawer”.
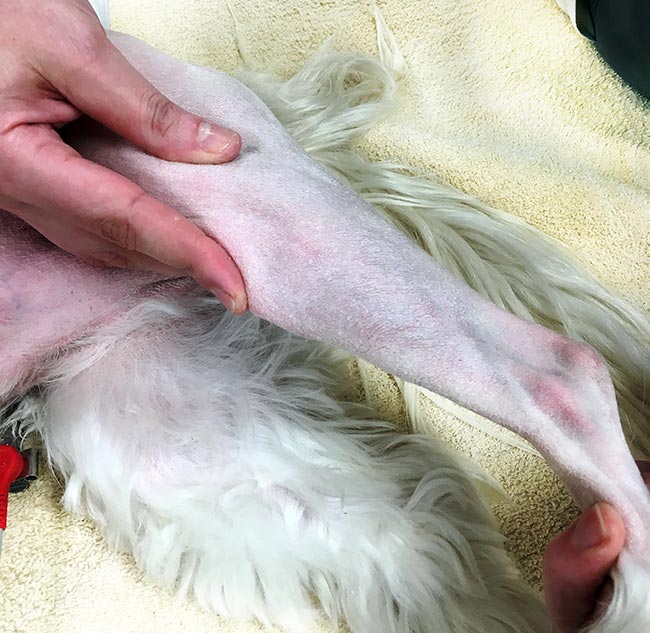
As described by Piermattei et al (2006), a tibial compression test is performed by placing the index finger of one hand over the length of the patellar ligament, starting at the distal aspect of the femur to reach the tibial tuberosity. The paw is held with the opposite hand and dorsiflexed as far as possible then relaxed (Figure 4). If drawer movement is present, forward movement of the tibial tuberosity is detected by the tip of the index finger resting on it. Unlike direct cranial drawer, this manoeuvre produces minimal pain and can be performed more easily in the conscious animal.
As mentioned previously, the CrCL is composed of two different functional bands: the CrM band is taut in both flexion and extension of the stifle, while the CdL band is taut only in extension (Arnoczky and Marshall, 1977). With this concept in mind, it becomes easier to understand why both direct and indirect cranial drawer should be assessed throughout the full range of motion of the stifle joint and why some partial tears may not elicit drawer motion. Indeed, if the CrM band only is affected and the CdL band is intact then a small amount of drawer will be appreciable in flexion (when the CrL is relaxed), but not in extension (when the CrL is taut).
Conversely, damage to the CdL band only will not lead to any drawer motion at any stifle position, as the remaining intact CrM band will be taut in both flexion and extension (Arnoczky and Marshall, 1977; Strom, 1990). In cases with partial CrCL tears, a pain response is often evident on full extension of the stifle, presumably due to stress on the damaged ligament as it becomes taut in this position.
Other factors that might limit drawer motion, despite the presence of CrCL pathology, include periarticular fibrosis in chronic cases, and painful and tense animals with increased muscle tone. Hence, while a positive drawer motion (whether direct or indirect) always indicates CrCL disease, its absence does not rule it out. In these challenging cases where history, clinical signs and orthopaedic examination alone do not allow us to reach a definitive diagnosis, radiographs of the stifle become particularly helpful.
Stifle radiographs are warranted in all cases to document the degree of OA in routine cases, to confirm stifle pathology in challenging cases with partial tears and to rule out other disorders, such as osteochondrosis, fracture or neoplasia (Kowaleski et al, 2012).
A minimum database of caudocranial and mediolateral views of the stifle should be obtained and the animal is typically sedated to obtain diagnostic views. Obtaining appropriately positioned views is important to facilitate interpretation and radiographs may be used subsequently for surgical planning.
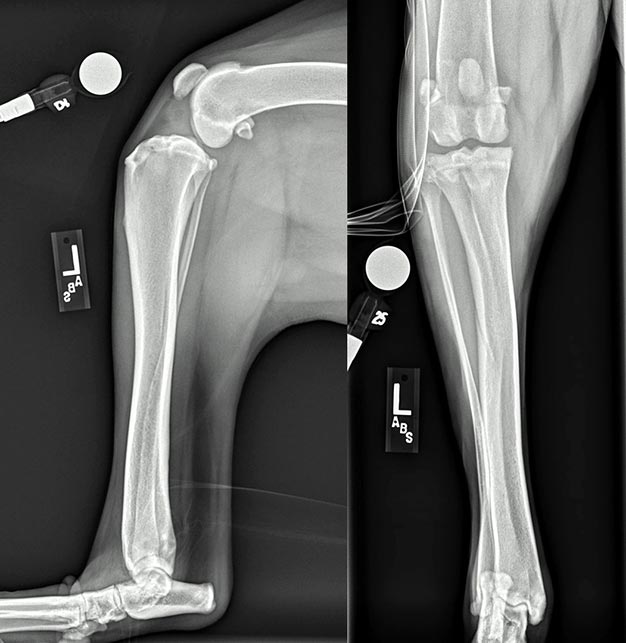
Radiographic safety protocols and legislation must be followed at all times. Before antagonising sedating agents, the stifle should be assessed for instability in cranial drawer in both flexion and extension. In cases where this was previously not appreciated due to a tense or nervous patient, obvious laxity may be apparent. In cases with partial tears, however, instability and a definitive diagnosis may remain elusive. Radiographs (Figure 5) are assessed for the following (Piermattei et al, 2006; Harasen, 2002; Kowaleski et al, 2012):
The infrapatellar fat pad is situated intracapsularly and extrasynovially in the stifle. On a lateral projection of a normal stifle, the infrapatellar fat pad shadow is represented by a triangle of dark lucency just caudal to the patellar tendon. This area of lucency extends from the distal aspect of the patella proximally, to the tibial crest distally, and to the origin and insertion of the CrCL caudally. The cruciate ligaments and menisci account for the normal radiodensity just caudal to this area. The earliest and most consistent finding with CrCL pathology is loss or effacement of the fat pad shadow by a soft tissue opacity. With CrCL pathology, synovial effusion and/or fibrosis of the fat pad region develop and gradually obliterate this area of dark lucency from caudal to cranial as synovial fluid, fibrosis or both accumulate. Typically, the area cranial to the femur becomes more radiopaque.
Osteophytes are most commonly seen around the distal patella, the supratrochlear region, the tibial and femoral margins, and the fabellae. When present, enthesiophytes are seen at the apex of the patella as well as at the origin and/or attachment of the CrCL (for example, at the level of the intercondylar fossa and within the cranial intercondyloid area of the tibia, respectively).
Even in the presence of CrCL rupture, the unstressed leg should lie in a neutral drawer position. If cranial translation of the tibia in relation to the femur is obvious then meniscus injury, such as a meniscus wedging the tibia forward, should be suspected.
Avulsion of the CrCL is an uncommon condition of skeletally immature dogs. Physical examination findings are similar to those described for rupture of the CrCL, except both drawer motion and joint effusion are marked. Radiographs demonstrate the avulsed bone fragment in the intercondylar space (Figure 1 of part 1).
Cases where CrCL disease is suspected, but cannot be definitively confirmed due to the absence of laxity in cranial drawer, can represent a diagnostic challenge. While advanced imaging has been reported in pursuing a definitive diagnosis in these cases where a high index of suspicion exists and the owners are keen to pursue surgical stabilisation, the authors advocate exploratory arthroscopy or arthrotomy to achieve a diagnosis.
Joint exploration is an integral component of surgical treatment for this condition regardless, as will be discussed in part three, and, following this, stabilisation of the stifle can proceed routinely, without the need for additional expensive tests or subsequent anaesthetic episodes. In cases where the index of suspicion remains low, certainly additional diagnostic tests may be required prior to surgical exploration.
Part three of this article will present treatment options: indications for surgical stabilisation will be considered in addition to the prognoses associated with the different treatment options.