27 Feb 2024
Chris Dixon covers diagnosis, treatment and management of this common ophthalmic condition in pets.

An eye slit lamp in use.
Corneal ulcers are a common and potentially serious ophthalmic condition in both human and veterinary medicine. They can result from a variety of causes and can lead to significant discomfort, vision impairment and even blindness if not promptly, and appropriately, managed.
The effective diagnosis and treatment of corneal ulcers is paramount to the successful resolution of the disease, and this article will explore ongoing research aimed at improving our management techniques.
The cornea is a transparent, avascular structure forming the anterior wall of the eye. The cornea is the principal refractive structure of the eye; in dogs, the cornea is approximately 0.5mm to 0.6mm thick, with the central region being slightly thinner than the periphery (Gilger et al, 1991).
The majority of patients with corneal ulceration will demonstrate signs of ocular discomfort which may include: increased blink rate, blepharospasm, third-eyelid protrusion and excessive tear production.
It is essential to identify or eliminate any potential causes of corneal ulceration, as internal and external factors can influence the management of the disease. Particular attention should be focused on any potential source of mechanical abrasion.
Common causes of ulceration include:
Multiple studies have reported that brachycephalic breeds are at a higher risk of developing corneal ulceration compared to non-brachycephalic breeds. James-Jenks et al (2023) demonstrated that brachycephalics are also more likely to have a diagnosis of complex corneal ulceration – particularly Descemetocele development. Several authors have suggested this is due to the combination of exaggerated morphological features, reduced corneal innervation and impaired healing capacity.
All ophthalmic examinations should start with hands-off assessment of the patient, paying particular attention to the periocular structures as a potential source of mechanical abrasion. A Schirmer tear test (STT) should be performed to assess reflex tear production of both eyes, with a normal range being 15mm/min to 25mm/min. It is common for cases with corneal ulceration to generate elevated STT readings, and this test should be avoided if evidence of deep corneal ulceration is present.
The combination of magnification and a focal light source is required for detailed examination of the cornea. Although more commonly used for examination of the retina, turning the lens wheel on a direct ophthalmoscope to +20D will provide a highly magnified illuminated image. If the direct ophthalmoscope has the option to generate a slit of light, this may help the examiner to gauge the depth of the ulcer. Ophthalmologists will use a binocular slit-lamp biomicrosope to provide a cross-sectional view of the cornea (Figure 2), but these delicate instruments are expensive and not commonly found in general practice clinics.
Only a very small quantity of fluorescein is required to stain a corneal ulcer, and ophthalmologists will often wash the corneal surface with sterile water to avoid over-saturation of the dye following its application. Fluorescein is hydrophilic and will highlight any exposed stromal tissue. Fluorescein will not stain the hydrophobic corneal epithelium or Descemet’s membrane.
Antibiotics play a crucial role in managing corneal ulcers, due to the risk of opportunistic infection. Antibiotic selection should be dictated by the type of infection and its likely susceptibility to an antibiotic class while following the prescribing cascade.
Multiple published reports have found that the most common organisms found in infected corneal ulcers are Gram-positive cocci (Staphylococcus or Streptococcus species) or Gram-negative rods (Pseudomonas species). In-house cytology can determine the type of bacteria present before bacteriology results are processed by a laboratory, and provide a head-start when considering an appropriate antibiotic.
Corneal ulcers can be extremely painful, and it is not uncommon for multiple medications to be prescribed when managing both infection and pain. Jost et al (2022) studied the effect of combination therapies on corneal healing and reported that an increased number of topical medications resulted in longer healing times. Multiple authors have noted that preservatives such as benzalkonium chloride, commonly found in topical ophthalmic medications, are epitheliotoxic, and the negative effect on corneal re-epithelialisation may be additive.
The placement of a soft bandage lens can provide mechanical protection to the cornea, and research has shown they may increase comfort and reduce the time taken for a corneal ulcer to heal.
Bandage contact lenses are less likely to be retained on an irregular corneal surface, and should not be applied if evidence of deep corneal ulceration is present.
Superficial corneal ulcers are common and only involve the outermost layer of the cornea, the corneal epithelium. Uncomplicated superficial ulcers will usually heal within three to seven days, with epithelial cells migrating from the limbus in a centripetal pattern. Cenedella and Fleschner (1990) reported that complete replacement of the corneal epithelium can occur within two weeks.
Superficial ulcers are typically less severe than deep ulcers, but are more painful due to exposed sensory nerve fibres. Treatment should include a topical broad-spectrum antibiotic to prevent opportunistic infection by the residential conjunctival flora, and consideration for analgesia with oral NSAID medication.
If evidence of reflex uveitis (severe pain, miotic pupil, aqueous flare) is present then the application of topical atropine 1% may be considered.
Topical local anaesthetics (such as proxymetacaine or tetracaine) should not be dispensed due to their epitheliotoxicity, and may significantly delay healing or allow for severe secondary complications.
If a superficial ulcer fails to heal within an expected time frame (21 days), the cornea should be evaluated for the possibility of a spontaneous chronic corneal epithelial defect (SCCED). This condition is most common in dogs older than seven years of age and occurs as a result of a failed attachment between the epithelium and stroma.
Research continues into the exact mechanism of the pathophysiology, and Bentley et al (2001) demonstrated the presence of a deficient epithelial basement membrane and hyaline acellular zone in the superficial stroma, preventing normal adhesion of the epithelium. A recent paper by Meurs et al (2021) has identified a gene mutation (NOG) in the boxer dog that may explain a predisposition to SCCED development in this breed. The condition can be diagnosed if fluorescein dye highlights under-running of the epithelium (Figure 3).
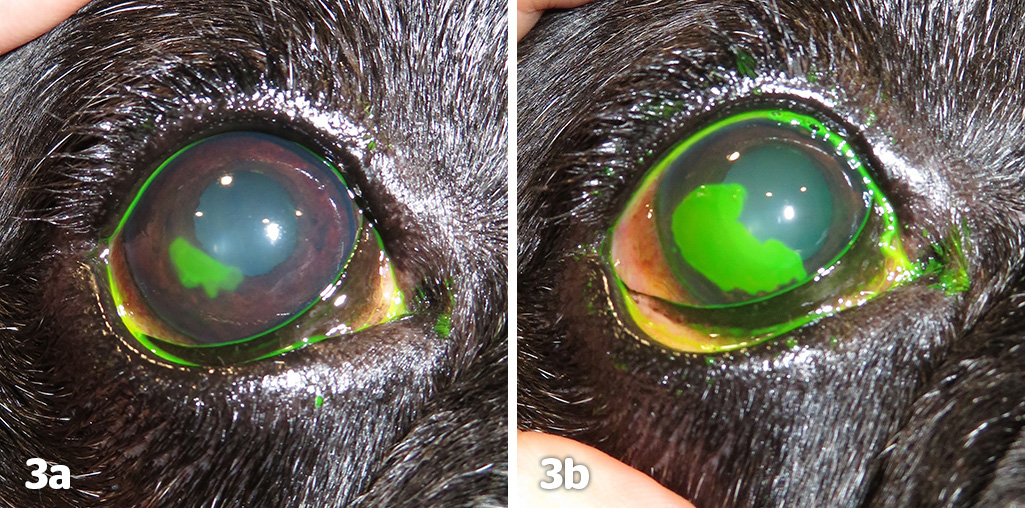
To facilitate corneal healing, the loose epithelium must be removed and the abnormal basement membrane destroyed. A number of different techniques exist and they broadly fit into two categories: keratotomies and keratectomies, with the most common listed in Table 1.
| Table 1. A comparison of published techniques for the management of spontaneous chronic corneal epithelial defect | ||||
|---|---|---|---|---|
| Technique | Number of combined cases from the published reports | Mean success rate from the published reports | Mean healing time from the published reports | Published reports |
| Grid keratotomy | 417 | 90.86% | 12.23 days | Boutin et al (2020) Spertus et al (2017) Stanley et al (1998) Wooff and Norman (2015) |
| Diamond burr keratotomy | 548 | 80.95% | 14.2 days | Gosling et al (2013) Hung et al (2020) Spertus et al (2017) Wu et al (2018) |
| Superficial keratectomy | 28 | 100% | 11.65 | Peiffer et al (1976) Stanley et al (1998) |
The loose corneal epithelium is removed by debridement, which can be achieved in a conscious patient following the application of a topical local anaesthetic.
Debridement can be performed with a sterile cotton bud, and the repeat application of fluorescein dye will confirm no further epithelial under-running. To remove the hyaline acellular zone in the superficial stroma, a keratotomy can be performed using a needle (grid or punctate pattern), a low-temperature thermocautery instrument or a diamond burr (Figure 4).
Repeat corneal debridement and keratotomy should not be performed until the cornea has had sufficient time for re-epithelialisation to occur.
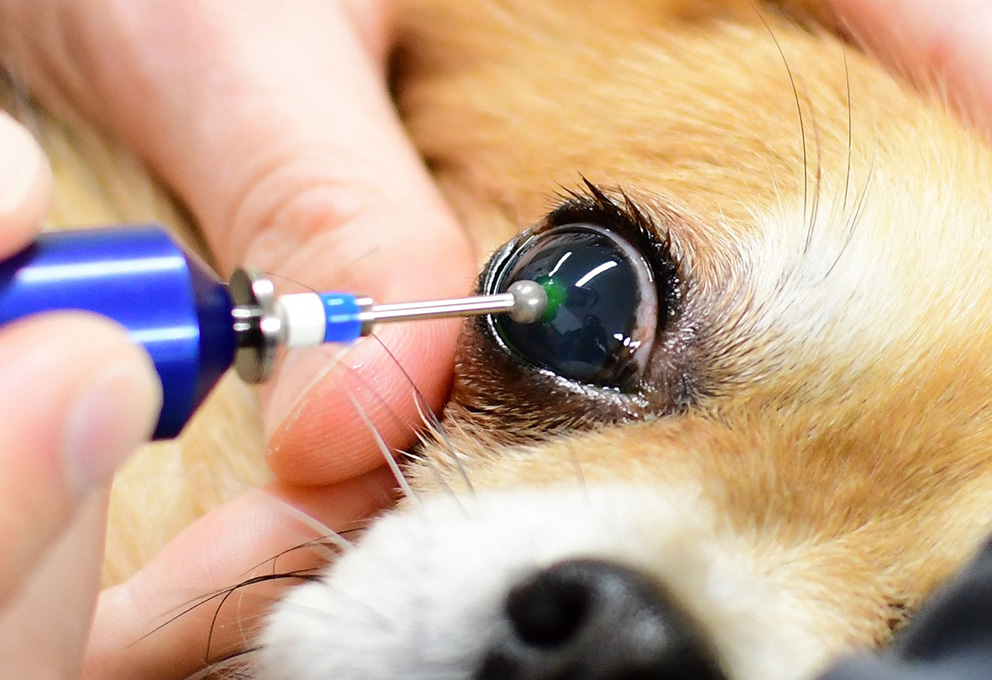
A superficial keratectomy requires general anaesthesia, an operating microscope and microsurgical expertise.
Keratectomies remove the epithelium and anterior stroma, and published reports suggest a success rate of 100%, and rapid healing. In referral practice, it is not uncommon for a patient to be presented for a superficial keratectomy if a keratotomy has failed to facilitate complete healing of the SCCED.
Infected corneal tissue can appear off-white or yellow, and may have associated oedema and vascularisation within the surrounding tissue.
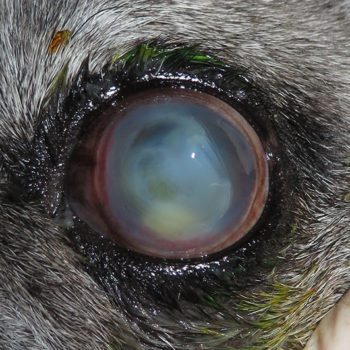
Keratomalacia (melting ulcer) is the degeneration and liquefaction of the stroma caused by proteolytic enzymes, leading to a rapid loss of normal corneal architecture (Figure 5). Studies have demonstrated that infection with Pseudomonas aeruginosa and β-hemolytic Streptococcus species are most likely to be associated with keratomalacia (Guyonnet et al, 2020; Tsvetanova et al, 2021).
Enzymes are released by both bacteria and corneal cells (epithelial cells, fibroblasts, leukocytes) in response to a corneal injury, and emergency medical management should be aimed at inhibiting proteolysis. In addition to appropriate antibiotic selection and analgesia, the following protease inhibitors should be considered:
In the author’s experience, refrigerated topical serum or plasma can be effective when applied frequently (every two hours), but caution should be given to the epitheliotoxicity of EDTA.
Ointments should be avoided when treating deep corneal ulcers, and tetracyclines (such as oxytetracycline or doxycycline) can be given orally to inhibit matrix metalloproteinase activity.
Corneal cross-linking (CXL) is a therapeutic technique that can increase the number of covalent bonds between the collagen fibrils within the corneal stroma. This is achieved by exposing riboflavin (vitamin B2) soaked collagen to a controlled beam ultraviolet light.
CXL not only increases the biomechanical and biochemical stability of the cornea, but has been shown to have an antimicrobial effect.
Several studies in the veterinary literature have shown that CXL is effective at arresting corneal melting and stabilising the cornea when treating cases with keratomalacia. CXL should be performed under general anaesthesia to ensure accurate irradiation of the target tissue, and it can be combined with corneal graft procedures if additional tectonic support is required.
With increased reports of drug-resistant microorganisms, the antimicrobial effects of CXL may become more important in the future.
The loss of corneal stromal tissue can threaten the structural integrity of the eye and lead to rupture of the globe. Early diagnosis and prompt treatment is critical to successful management of these cases – especially when considering the possibility of active infection within the cornea.
Descemet’s membrane is transparent, extremely thin (31µm to 12µm) and fragile. A Descemetocele is the deepest possible corneal ulcer before perforation occurs, and it is a surgical emergency (Figure 6).
A corneal graft should be considered if the stromal loss is greater than 50% of the total corneal thickness and the ulcer threatens the structural integrity of the eye.
Corneal surgery requires microsurgical skills and instrumentation, and the type of corneal graft will depend on the size and location of the ulcer. Autografts, allografts and xenografts are commonly used by ophthalmologists to replace damaged corneal tissue. Recent research has contributed to advancements in surgical techniques and materials, and studies have explored the outcomes of various grafting methods.
A corneoconjunctival transposition is a sliding lamellar keratoplasty frequently used by veterinary ophthalmologists to repair deep corneal ulcers.
A section of healthy tissue is transposed from the adjacent cornea and fixed into position with 9/0 to 11/0 suture material. Transposition of the connected limbus and conjunctiva improves viability of the tissue, and reduces the distance required for cellular migration. If the healthy tissue is insufficient to transpose, a commercially available xenograft may be considered to provide tectonic support, but reaction to the material may increase postoperative corneal scarring.
Ruptured corneal ulcers represent some of the most challenging cases in veterinary ophthalmology. They can occur when untreated corneal ulcers lead to a full-thickness perforation of the cornea.
Ruptured ulcers are painful due to decompression of the anterior chamber, and exposure of the intraocular environment can lead to endophthalmitis. Immediate surgical repair with a graft technique is required to stabilise the globe, and iris repositioning may be required if prolapse has occurred (Figure 8).
The effective management of corneal ulcers in domestic animals is a multifaceted challenge which requires rapid identification of an underlying cause, determination of ulcer depth, as well as consideration of primary or opportunistic infection; it demands expertise if microsurgical repair is to be considered.
Ongoing research in veterinary ophthalmology continues to advance our understanding and treatment options for corneal ulcers, ultimately improving the quality of care we can provide to our patients.