26 Mar 2024
Jordi Balana Pedrol and Emily Dutton provide readers with an overview of both the aetiology and available treatment options for canine patients presenting with this condition.

Myxomatous mitral valve disease (MMVD) is the most common acquired heart disease in dogs in many parts of the world, and its progression until onset of congestive heart failure (CHF) is somewhat unpredictable1.
Dilated cardiomyopathy (DCM) is the second-most common acquired heart disease in dogs, a known cause of CHF in the species and a major cause of sudden death in a range of medium to large-breed dogs.
Certain breeds are over-represented in the UK, with DCM being suspected to be familial or genetic in the Dobermann, boxer, deerhound, great Dane and Irish wolfhound (Figures 1 and 2). Smaller breeds are sometimes affected, such as the cocker spaniel2.
This article is focused on the most common acquired causes of CHF; therefore, congenital causes are not discussed. Management and treatment of canine chronic CHF has changed substantially over the past decades3. This article is intended to provide an up-to-date review of current treatment options for chronic CHF (stages C and D MMVD and DCM) and its supportive care.
A survival benefit of pimobendan administration and prolonged time until onset of CHF or cardiac-related death when compared to patients not receiving this medication has been shown in the preclinical phase of both DCM4 and MMVD5.
Pimobendan use in patients with CHF, when added to conventional therapy, has shown increased time to cardiac-related death or treatment failure when compared with benazepril6,7, and also greater improvement in short-term cardiac function6.
Treatment with pimobendan in patients with CHF has prolonged survival times when compared to a control group in both a low dose (dose range: 0.05mg/kg to 0.19mg/kg every 12 hours) group and a standard dose (dose range: 0.20mg/kg to 0.48mg/kg every 12 hours) group8. Dogs receiving standard dose in that study had a significantly lower recurrence rate of pulmonary oedema when compared with the low-dose pimobendan and the control group. Continuation or initiation of pimobendan therapy in patients with CHF would be recommended in view of the shown benefits of treatment (Figures 3 and 4).
Angiotensin-converting enzyme inhibitors (ACEIs) suppress the renin-angiotensin-aldosterone system by inhibiting the angiotensin-converting enzyme and decreasing the formation of angiotensin II; therefore, inhibiting sodium retention, vasoconstriction, systemic hypertension and myocardial remodelling.
Theoretically, these are beneficial in the treatment of heart failure9. Enalapril showed an improvement in clinical signs and quality of life in dogs with CHF due to MMVD and DCM in the Cooperative Veterinary Enalapril (COVE)10 and Invasive Multicenter Prospective Veterinary Evaluation of Enalapril (IMPROVE)11 studies.
In another study (Long-Term Investigation of Veterinary Enalapril; LIVE), dogs with CHF due to DCM and MMVD receiving enalapril improved clinically or stabilised for a significantly longer period when compared to dogs not receiving ACEIs12. Benazepril showed statistically significant prolonged survival times and improved clinical signs in the Benazepril in Canine Heart disease (BENCH) study. However, due to small numbers, significance was lost for the DCM group when studied alone13.
In the more recent Vasotop, Lasix and Vetmedin (VALVE) study, the addition of the ACEI ramipril to pimobendan and furosemide treatment did not show any benefit on survival time in dogs with CHF due to MMVD14. Therefore, the design of some of the older ACEI studies have been debated.
More recently, suggestions have been made that the ACEI dose used in some of the previous canine studies was too low. In fact, in a more recent retrospective study15, a higher ACEI dose was associated with increased survival time from first onset of CHF. A higher ACEI dose in patients with CHF was associated with improved survival at two years.
Starting or continuing benazepril or enalapril at 0.5mg/kg by mouth twice daily (or other ACEIs at an equivalent dose), and measuring serum creatinine and electrolytes three to 14 days after commencement of treatment, has been recommended16.
The design of some of the older studies have been criticised, but many cardiologists still advocate their use.
| American College of Veterinary Internal Medicine classification of myxomatous mitral valve disease (MMVD) in dogs16 | |
|---|---|
| Staging of MMVD in dogs | |
| Stage A | Dogs at high risk for developing MMVD, but that currently have no identifiable structural disorder of the heart (no audible heart murmur). |
| Stage B1 | Asymptomatic dogs with structural MMVD, that have no (or mild) radiographic or echocardiographic evidence of cardiac remodelling. |
| Stage B2 | Asymptomatic dogs with structural MMVD, with radiographic and echocardiographic findings of left atrial and ventricular enlargement. |
| Stage C | Dogs with either current or past clinical signs of heart failure caused by MMVD. |
| Stage D | Dogs with end-stage MMVD, in which clinical signs of heart failure are refractory to standard treatment. |
Spironolactone is an aldosterone antagonist that reduces myocardial and vascular endothelial fibrosis, as well as being a weak potassium-sparing diuretic. A moderate level of evidence exists for its use. It showed a significant reduction in morbidity and mortality when added to conventional treatment of CHF in patients with MMVD, with a lower percentage of patients reaching cardiac endpoint and showing a greater overall survival probability when receiving this drug17, 18.
The evidence is limited though as, with the first study, some of the selected patients were thought to be coughing due to respiratory disease instead of CHF, so different ACEIs were used at varying doses and pimobendan was not prescribed. The effect of spironolactone on survival in dogs with CHF due to DCM was not examined.
Addition of spironolactone to benazepril during the preclinical phase of MMVD (stage B2) failed to demonstrate a delay in onset of CHF in the Delay of Appearance of Symptoms of Canine Degenerative Mitral Valve Disease Treated with Spironolactone and Benazepril (DELAY) study19.
In all these studies, patients received doses ranging from 1.99mg/kg/d to 4mg/kg/d. Lower doses (of 0.49mg/kg/d to 0.8mg/kg/d) showed no significant effect on survival times in dogs with CHF at three and six months after initiation of the treatment20. Addition of spironolactone to standard therapy for CHF did not show a survival time benefit in Dobermanns with DCM; although, atrial fibrillation (AF) was significantly less frequent in the patients receiving this drug21.
The owners of the dogs in this study, however, were not specifically instructed to administer the spironolactone with food. This may have affected the drug’s efficacy, as it is known that the oral bioavailability of spironolactone is significantly improved from 50% to 80% to 90% with administration of food. Some possible reasons for the reduced incidence of AF in the spironolactone group may be due to its anti-fibrotic effects, as well as that it may block electrical and cardiac remodelling. Use of spironolactone in large-breed dogs with DCM is off licence.
Loop diuretics are the cornerstone of CHF therapy and are used in the management of both acute and chronic CHF in dogs with MMVD. With chronic CHF, oral furosemide treatment is used to effect to maintain patient comfort, usually at doses of 2mg/kg every 12 hours, and monitoring of creatinine, blood urea nitrogen and electrolytes concentrations are advised 3 to 14 days after initiation of diuretic treatment16.
Studies showing a mortality benefit with furosemide are lacking and, given ethical implications, it is unlikely this type of study would go ahead. Clinical trials have shown that torasemide is non-inferior to furosemide for treatment of CHF due to MMVD22, 23. Furthermore, the Canine Relief of Pulmonary Oedema by a DIuretic Easy Management (CARPODIEM) study showed a significantly decreased risk of reaching spontaneous cardiac death or premature withdrawal from the study for cardiac reasons22.
To the authors’ knowledge, no studies exist comparing the use of furosemide and torasemide long term.
The effective dose of torasemide has been reported to be 10% the furosemide diuretic dose in dogs24. In another study including healthy dogs25, diuresis and natriuresis observed with torasemide were similar to those obtained with furosemide at dose ratios from 1/10 to 1/20. A torasemide dose of 5% to 10% the furosemide effective dose is recommended for chronic management of CHF16.
| Classification of dilated cardiomyopathy (DCM) in dogs | |
|---|---|
| Staging of DCM in dogs | |
| Stage A | Dogs of predisposed breeds at risk of DCM with no cardiac disease detectable by echocardiography or Holter. Dogs with positive genetic tests, but without detectable evidence of DCM. |
| Stage B1 | Dogs without symptoms of congestive heart failure (CHF) where syncope is possible, with either arrhythmias related to DCM, but without echocardiographic evidence of DCM. May have atrial fibrillation or ventricular arrhythmias. |
| Stage B2 | Dogs without symptoms of CHF where syncope is possible, with detectable DCM by echocardiography, with or without arrhythmias. |
| Stage C | Past or current clinical signs of objectively documented CHF due to DCM. |
| Stage D | Dogs with end-stage refractory CHF due to DCM, in which clinical signs of heart failure are not responding to maximal or optimum medical treatment. |
Different surgical interventions to repair the mitral valve apparatus in dogs have been described26-28. For patients with CHF due to MMVD, mitral valve repair surgery may be recommended in centres with low complication rates16. However, the cost of the surgical procedure, its invasive nature and the limited number of centres offering it are limiting factors for many patients29.
Alternative transcatheter edge-to-edge mitral valve repair has been documented in healthy dogs29 and dogs with stage B1 MMVD30, but to the authors’ knowledge, no published survival or outcome studies are available.
In cases where fast AF occurs as a complication of atrial stretch due to MMVD, the aim should be heart rate control with negative chronotropic medications to a mean heart rate of less than 125 beats per minute. This has been shown to improve survival times in dogs with AF31.
Rate control can be achieved with use of different medications (including calcium channel blockers, beta blockers, digoxin or potassium channel blockers) or their combination32. The combination of digoxin and diltiazem showed greater efficacy at lowering the heart rate when compared to either drug alone for dogs with AF33.
Initiation of a beta blocker in patients with clinical signs of CHF due to MMVD is not recommended due to its negative inotropic effects. However, if a patient had already been receiving a beta blocker before onset of CHF, treatment may be continued with consideration of reducing the dose in the face of signs of low cardiac output, hypothermia or bradycardia16.
Monitoring of these patients is essential to control the progression of the disease. Sleeping respiratory rate has been shown to be a sensitive and specific tool for identifying CHF as a cause of respiratory signs34. The aim for patients with chronic CHF is for the sleeping respiratory rate to be less than 30 breaths per minute in the home environment35. Commonly, refractory CHF (stage D) is associated with cardiac cachexia in dogs36, 37 and is associated with shorter survival times38. These animals can be overweight, but still have significant muscle loss. Monitoring muscle condition score can, therefore, be especially useful in CHF patients39.

Obesity is a risk factor for the development of CHF in people, but it is also associated with a cardioprotective effect once CHF is present40. Cardiac cachexia is a multifactorial problem that results from decreased food intake, increased energy requirements and an increased production of inflammatory cytokines. In dogs with CHF, weight gain and obesity have been shown to be associated with longer survival41. In dogs that gained weight during the course of their disease, they had a longer survival time than those that lost or maintained weight41.
Omega-3 fatty acids are available in fish oil sprays and capsules, and result in decreased production of cytokines and inflammatory mediators. The fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) may have beneficial effects on cardiac cachexia associated with DCM, as well as possibly increasing appetite42. They may also have anti-arrhythmic and anti-inflammatory properties40, 42-44.
Plasma fatty acid abnormalities and their normalisation after omega-3 fatty acids supplementation have been reported in dogs with CHF42. In a more recent prospective study45, improved echocardiographic, radiographic, electrocardiographic and Holter analysis parameters were seen in dogs with stages B2 and C MMVD that received oral omega-3 fatty acids when compared to those who did not receive them. Supplementation of omega-3 fatty acids should be considered in patients with CHF – especially in cases where arrhythmias, cardiac cachexia or decreased appetite are present16. Recommended oral doses are EPA 40mg/kg EPA and 25mg/kg DHA, daily.
Recommendations of 60Kcal/kg bodyweight of calorie intake to minimise weight loss have been suggested16, but patients should be fed according to their individual characteristics and diet requirements46. Protein intake is important in patients with CHF, and no evidence exists of benefit from restricted protein intake. Use of renal diets with reduced protein content should, therefore, be avoided unless concurrent severe renal dysfunction is present37, 46.
Sodium-restricted diets have been used in patients in stage D CHF, despite the lack of research of their use in these patients, until a study showed significant reduction in cardiac size measures in patients receiving low sodium diets when compared to a dog receiving medium sodium content diets47.
Low sodium diets are generally unpalatable, and so are not usually recommended due to that reason alone. Although a paucity exists of long-term studies to support benefits on survival time or disease progression, recommendation of diets with sodium content less than 80mg/100kcal have been suggested46.
It is important to include treats, table food, food used to administer medication or any other sources of food when considering sodium intake.
As described previously, electrolyte imbalances, such as hypokalaemia, can be seen with diuretic administration. On the other hand, hyperkalaemia is theoretically possible in patients receiving ACEIs and spironolactone; although, this is rarely documented in practice. Therefore, serum electrolyte levels monitoring is important and, if identified, hypokalaemia should be treated with oral potassium supplements. L-carnitine deficiency has been associated with DCM in dogs48, 49. Some patients improved after L-carnitine supplementation, but some of them also received concurrent taurine supplementation49-51. L-carnitine supplementation could be beneficial even in cases where L-carnitine deficiency is not present – especially in boxers and cocker spaniels – although costs and possible gastrointestinal side effects should be taken into consideration.
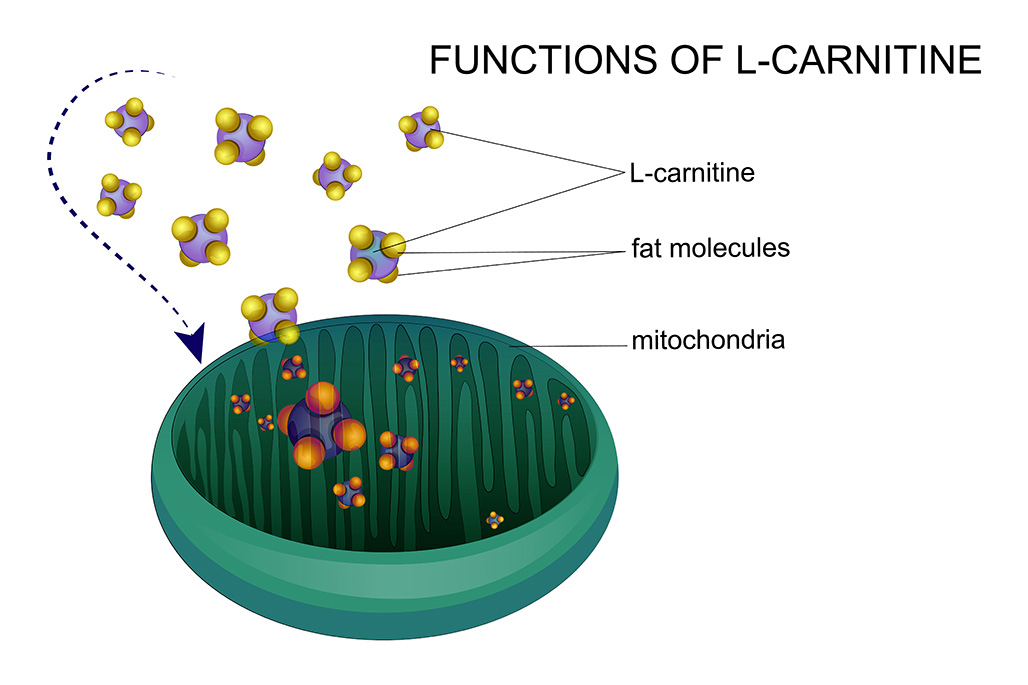
L-carnitine is a compound found abundantly in meat-based diets or produced endogenously from the amino acids methionine and lysine. Among its key functions is the transportation of long-chain fatty acids across the mitochondrial membrane to facilitate beta-oxidation and, ultimately, adenosine triphosphate production. Blood concentrations of L-carnitine do not always correlate with myocardial levels, making designing good prospective studies difficult.
Taurine deficiency has also been reported as a nutritional form of DCM49, 53-56 that improved after taurine supplementation; although, in some of these cases, diet changes were also made, making it difficult to interpret if the improvement was due to diet change or taurine supplementation.
Taurine can be produced endogenously in dogs, but not cats, from the amino acids methionine or cysteine. It is found in meat, fish or dairy products. Taurine is an anti-oxidant, anti-inflammatory, a membrane stabiliser, a positive inotrope, important for osmoregulation, is anti-renin-angiotensin-aldosterone system, and important for retinal health and glucose regulation.
In 2018, the Food and Drug Administration reported a potential connection between DCM and non-traditional diets containing a high proportion of peas, lentils, other legume seeds or potatoes as main ingredients (pulse-rich diets) – many labelled as “grain free”57. Some studies have investigated this possible association and have shown that dogs with DCM that had been fed a grain-free or pulse-rich diet had clinical and echocardiographic improvement after diet change to a grain-inclusive diet, suggestive of a degree of reversibility to the nutritional cardiomyopathy58-60. One study60 looked only at golden retrievers and all of the dogs had low taurine levels; therefore, they were supplemented with it.
The other two studies included different breeds and found that low-circulating taurine concentrations was uncommon, suggesting that taurine deficiency was not the cause for development of nutritional cardiomyopathy. Survival was significantly longer in dogs eating a non-traditional (grain-free) diet and then switched to a traditional (grain-inclusive) diet compared to those dogs that did not have a diet change and remained on grain-free diets59. This also applied when a sub-sample of patients with CHF was analysed.
Dogs that received taurine supplementation also had longer survival times in comparison to those that did not receive it, regardless of their plasma or whole blood taurine concentration59, showing benefit of taurine supplementation.
Taurine supplementation improved left ventricular systolic function and reduced left ventricular dimensions in English cocker spaniels with DCM and taurine deficiency61. This benefit could be due to taurine’s anti-oxidant and positive inotropic effects62. Starting taurine supplementation in cases of CHF due to DCM can be beneficial regardless of their plasma or whole blood taurine levels52, but the stress associated with polytherapy and additional cost must be taken into account.
In view of the possible association between grain-free and pulse-rich diets and DCM, it would be advisable to restrict feeding these non-traditional diets to select cases, where the patients can only be fed these types of diets. In cases of patients presenting with DCM, regardless of the presence or absence of CHF, it would be advisable to change their diet due to the evidence described.
Finally, the length of time that a dog has been fed a grain-free diet has also been shown to be important in one study where survival was inversely related to the length of time eating grain-free food before DCM diagnosis63. The dogs eating grain-free diets for a shorter amount of time lived significantly longer than those dogs that were fed grain-free diets for longer.
Reports exist of other diets (vegan, vegetarian, protein-restricted, lamb meal and rice diets, plus kangaroo or bison diets) that have also been associated with diet-induced DCM55, 64.
Some were thought to have involved taurine deficiency, as the DCM reversed in those dogs after taurine supplementation. It was possible that the diets provided inadequate or unavailable amino acid precursors of taurine, which resulted in taurine deficiency.
The fibre, fat, or protein content of the rice bran may have accelerated excretion of bile acids, predisposing dogs to taurine deficiency. Relative to other meat meal sources, lamb meal has been shown to have poor ileal nitrogen and cysteine digestibility in dogs.
Ultimately, it is important to take a good history when assessing those patients with DCM, asking not only which diet has been fed, but also how long it has been fed for. Many questions regarding diet-associated DCM still exist that need answering, as the aetiology remains unknown.
However, we do know that diets high in legumes such as peas or lentils are associated with nutritional DCM.