21 Aug 2017
Martha Cannon, in the first of a two-part article, discusses guidelines and possible negative effects to look for regarding feline vaccines.

The widespread use of effective vaccinations against common infections has had a huge positive impact on the health of pet cats.
However, mounting evidence has indicated feline vaccinations can be associated with some significant – indeed, life-threatening – adverse effects, leading us to examine more closely the risk:benefit ratio of the traditional annual vaccination protocol.
The WSAVA Guidelines for the Vaccination of Dogs and Cats provides an in-depth review of evidence and advice pertaining to this controversial subject, and is free to access.
This two-part article highlights some of the main points raised by the guidelines. Part one reviews our understanding regarding adverse reactions to feline vaccination, while part two summarises the advice on how to use vaccinations to best effect, while minimising the risk of inducing disease.
The goal of any vaccination programme is to vaccinate as many “at-risk” animals as possible, using an initial primary vaccination course, followed by regular “boosters” to maintain effective immunity.
However, it is challenging to know how frequently booster vaccinations should be given because, while manufacturers have been required to demonstrate immunity is maintained through to their recommended booster interval, they have not been required to explore the total duration of immunity the vaccine induces. For many dog and cat vaccines, the recommended booster interval has been one year, although, for some agents, a longer interval has been established, such as feline panleukopenia vaccines and some rabies vaccines.
The recommendation for annual revaccination has fit well with a consensus view that pet dogs and cats should have an annual health check, and the booster has been a valuable prompt to encourage owners to bring their pets to the veterinary practice. Uncoupling the annual health check from an annual vaccination visit risks some owners ceasing to attend, which would be detrimental to their pets’ well-being, as well as to the income of the veterinary practice. However, as concern increases regarding the potential for vaccines to cause severe adverse effects in cats, it becomes harder to justify continuing to vaccinate all cats, against all agents, every year.
The WSAVA Vaccination Guidelines Group and the European Advisory Board on Cat Diseases are independent bodies that maintain up-to-date guidelines on vaccinations for cats – and, in the case of the WSAVA Vaccination Guidelines Group, also dogs. The advice from both bodies is largely the same and the overall concept is, as vets, we should:
Aim to vaccinate more individual cats
Aim to vaccinate each individual cat less often and only with antigens necessary for that individual
In the UK, core vaccines recommended for all cats provide protection against feline herpesvirus (FHV), feline calicivirus (FCV) and feline panleukopenia virus (FPV).
Vaccination against FeLV is considered a non-core vaccine, but is recommended for all cats that have free access to the outdoors. Rabies vaccines are required for cats that travel, but not otherwise. Other vaccines, such as those for Chlamydophila and Bordetella, are non-core vaccines and should only be used in cats at increased risk of the specific infection.
Vaccination against FPV and FeLV provides solid immunity for an extended period, but FHV and FCV vaccinations do not provide the same robust immunity:
Concerns have mounted regarding the potential for vaccinations to induce adverse effects. Allergic reactions and local irritation are the most commonly reported adverse effects (Moore et al, 2007), but feline injection site sarcoma (FISS) is also a well-documented problem (Hartman et al, 2015) and evidence has suggested the potential for feline vaccines to contribute to the development of chronic kidney disease (Lappin et al, 2006; Finch et al, 2016).
The reported incidence of suspected adverse reactions to vaccines is extremely low. In 2010, the VMD received 223 reports of suspected adverse reactions to feline vaccines (Dyer et al, 2011), but this accounted for nearly 50 per cent of all reported adverse reactions to feline-authorised products (the total number of reports received was 468).
Cats aged younger than or around one year old appear to be at highest risk of adverse vaccination reactions (The Cat Group, 2006; Moore et al, 2007) and, in one study from the US, risk increased with rising number of vaccine doses administered at one visit (Moore et al, 2007).
All suspected vaccine-associated sarcomas should be reported to the vaccine manufacturer and the VMD via the Suspected Adverse Reactions Surveillance Scheme (SARSS).
The majority of reported adverse reactions to vaccination arise within a few days (Moore et al, 2007). The most common reactions within this period are short-term, self-resolving lethargy and/or fever. Swelling, inflammation and soreness at the injection site mostly occurs within days to weeks of vaccination, and is usually self-limiting or responds well to topical symptomatic treatment.
Allergic reactions causing facial or periorbital oedema and generalised pruritus can also occur, but appear to be rare. In 2014, fewer than 50 cases were reported to the VMD via the SARSS. The reported incidence of anaphylaxis following vaccination in cats during the same period was similarly low (VMD, 2016).
In dogs, vaccinations have been implicated in the development of immune-mediated haemolytic anaemia (Duval and Giger, 1996), and possibly a variety of other immune-mediated disorders, such as myasthenia gravis and immune-mediated polyneuropathies, although evidence to support these is absent or based on single case reports. The 2004 “POOCH” study (Edwards et al, 2004) found no association between recent vaccination and ill health in dogs. No evidence links similar immune-mediated disorders with vaccination in cats.
Transient (usually two to three days duration) polyarthritis may develop following FCV vaccination (The Cat Group, 2006). The associated lameness varies in severity and often shifts from limb to limb. This problem is relatively rare and may be associated with concurrent infection with a field virus at the time of vaccination, rather than with an effect of the vaccine-strain virus itself.
While acute adverse reactions to feline vaccination are rare, and usually mild and self-limiting, more significant concerns exist regarding the potential for life-threatening chronic adverse reactions to vaccines in cats.
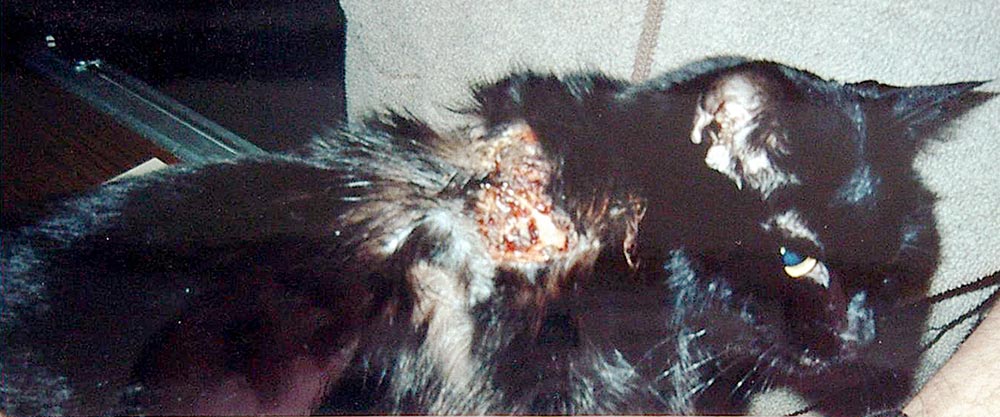
FISS is a rare, but genuine, phenomenon and many injectable products are documented to have caused FISS (Hartmann et al, 2015). The incidence of FISS in the UK per year has been estimated at between 1 in 16,000 to 1 in 50,000 cats registered with a veterinary practice (Dean et al, 2013).
Whether vaccines, and particularly adjuvanted (“killed”) vaccines, are more likely to induce FISS than other injections remains unclear, but because adjuvants promote local inflammation, they may have a role in tumorigenesis and WSAVA vaccination guidelines state “non-adjuvanted vaccines should be administered to cats wherever possible” (Day et al, 2016). However, acknowledging a low risk of FISS exists whichever vaccine is used, the site at which a vaccine is given should be one amenable to extensive surgical resection should a mass arise. For this reason, the interscapular region should be avoided, and the distal limbs and lateral abdomen are considered more suitable sites.
Rotating the site of vaccination from year to year should reduce the risk of inducing FISS. A practical way to achieve this is to adopt a practice policy stating the site at which feline injections will be administered during one calendar year, rotating through the recommended sites year by year, and recording on the clinical record the site of injection on each occasion (Day et al, 2016).
Where a mass does occur at an injection site (Figure 1) it should be investigated carefully at an early stage. If the mass is found to be malignant then radical, aggressive treatment should be used on the first occasion.
Frequent vaccination has been identified as a risk factor for the development of chronic kidney disease in cats (Finch et al, 2016). Vaccine strain viruses are grown in cell cultures and some use Crandell Rees feline kidney (CRFK) cells. During vaccine manufacture, CRFK contaminants remain in these vaccines and are subsequently injected into cats, stimulating production of auto-antibodies (Lappin et al, 2005) that bind to proteins in the kidney.
Auto-antibodies that bind to alpha-enolase and annexin-A2 have been identified in vaccinated cats; in humans, alpha-enolase antibodies can cause nephritis, and both alpha-enolase and annexin-A2 are associated with auto-immune disease (Whittemore et al, 2010). A small study of cats given multiple vaccinations over a two-year period induced interstitial nephritis in three out of six cats (Lappin et al, 2006).
Further studies are needed, but the concerns raised underline the importance of avoiding over-vaccination, while maintaining adequate protection for the individual and population.
In the majority of cats, the benefits of an appropriate lifetime vaccination protocol outweigh the risks of inducing an adverse effect. However, given adverse effects can and do occur, consideration needs to be given as to what comprises an “appropriate” protocol and over-frequent vaccination should be avoided.
Part two of this article will review advice on vaccination protocols aimed at providing continued good protection, while avoiding over-vaccination.