7 Jan 2019
Michael Groombridge describes the diagnosis and management of a dog that presented with apparent urinary incontinence.

Exposure of enlarged right external parathyroid gland.
Primary hyperparathyroidism is uncommon in dogs, but an important differential for persistent hypercalcaemia and associated clinical signs.
In this article, the author describes the workup and management of a case of primary hyperparathyroidism in first opinion practice. He discusses differentials for hypercalcaemia, how to differentiate between them, and the practicalities of diagnosis and management in the general practice setting.
A 13-year-old female, neutered, Jack Russell terrier presented due to drinking and urinating more than normal.
The owner also reported episodes of apparent urinary incontinence where the dog was urinating in the bed overnight.
Non-pathological causes
Pathological causes
Physical examination was unremarkable. A free-catch urine sample was analysed in-house – the urine specific gravity (USG) was 1.010 and nothing abnormal was found on the urine dipstick.
On sediment exam, moderate numbers of calcium oxalate monohydrate crystals were found, which can be dumb-bell shaped or have a picket fence appearance. Calcium oxalate monohydrate crystals can form in stored urine samples (Albasan et al, 2003). Pathological causes include urolithiasis, elevated calcium levels in the blood (Nelson and Couto, 2008) or can be seen with ethylene glycol toxicity (Jacobesen et al, 1982).
Due to the low USG, in addition to polyuria and polydipsia, a blood sample was taken to look for evidence of primary or secondary renal disease.
Relevant biochemistry results were:
The red figure is higher than the reference range, while the blue figure is lower. Haematology results were all within normal limits.
A decreased Na:K ratio can be seen with disease processes such as hypoadrenocorticism, diabetes mellitus and severe gastrointestinal disease. It has been shown lower Na:K ratios increase the probability of a diagnosis of hypoadrenocorticism, with 64% of cases with a Na:K ratio of less than 20:1 having hypoadrenocorticism (Neiger and Gunderson, 2003). No evidence of either diabetes mellitus (no hyperglycaemia or glucosuria) or gastrointestinal disease was present.
Hypercalcaemia can have a number of causes, which are summarised in Panel 1. Clinical signs are shown in Panel 2.
Ionised calcium is the active biological form. Total calcium is made up of ionised calcium (55%), calcium bound to albumin (35%) and calcium chelated to other compounds (10%; Schenck et al, 1996). Therefore, one should always bear in mind blood albumin levels when checking total calcium, as decreased albumin may give an artificially low total calcium reading.
The practice the author works in does not have the facility to run ionised calcium in-house, and neither does its usual reference laboratory. It therefore has to rely on total calcium measurements.
As hypercalcaemia can be due to artefact or laboratory error, a repeat sample was run one week later, and a urine sample sent for culture and sensitivity.
The urine culture result was greater than 105 colony-forming units/ml Escherichia coli. Co-amoxiclav (used under the cascade) was started at 12.5mg/kg by mouth twice a day based on the culture and sensitivity results.
Repeat biochemistry results were:
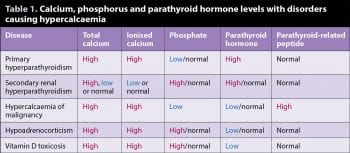
The repeat tests confirmed a persistent hypercalcaemia, as well as low phosphorus. The low phosphate made some of the differentials on the list of causes of hypercalcaemia less likely, including vitamin D toxicity, hypoadrenocorticism and secondary renal hyperparathyroidism, as with these disease processes, one would expect a high or normal phosphate level (Table 1).
The updated differential list for polyuria/polydipsia with hypercalcaemia and hypophosphataemia was:
Further physical examination, including a rectal exam, was performed to look for evidence of neoplasia, such as anal sac adenocarcinoma. This was found to be unremarkable. At this point, it was agreed with the owner to send samples externally to measure parathyroid hormone (PTH) and parathyroid-related peptide (PTHrP) levels, to help rule in/out some differentials.
What was not realised was the reference laboratory used by the author’s practice did not offer these tests and, therefore, one had to be found that did. PTH/PTHrP assay results were:
The persistent hypercalcaemia, hypophosphataemia and elevated PTH levels were strongly suggestive of primary hyperparathyroidism. Due to the elevated blood calcium levels, one would expect PTH to be in the lower half of the reference range in a normal dog due to negative homeostatic feedback on the parathyroid gland. Interestingly, it has been shown nearly 30% of dogs with primary hyperparathyroidism have UTIs (Feldman et al, 2005).
Renal system
Musculoskeletal system
Gastrointestinal system
Cervical ultrasound can be performed to look for enlarged parathyroid glands. Normal parathyroid glands should be less than 3mm in healthy dogs (Feldman et al, 2005).
In most cases, just one of the four parathyroid glands will be enlarged, but in approximately 10% of cases, two or more masses will be found.
The treatment of choice for management of primary hyperparathyroidism (PHPT) is surgical excision of the abnormal parathyroid glands.
Ultrasound-guided ethanol or heat ablation have also been described to treat PHPT, but have lower success rates than surgery (Rasor et al, 2007).
In addition, these options seemed less within the author’s competence range as a first opinion practitioner.
Hypercalcaemia can cause cardiac dysrhythmias including bradycardia as well as hypertension due to peripheral vasoconstriction. IV fluid therapy (Hartmann’s solution, 4ml/kg/hr) was instigated prior to surgery to help lower serum calcium levels.
Methadone (0.3mg/kg IV) was administered as premedication and for analgesia. Induction was with propofol to effect and maintenance was with isoflurane and oxygen.
A cervical ultrasound was performed before surgery with the aim of identifying the enlarged parathyroid gland(s), but locating it/them was unsuccessful. Non-invasive blood pressure was used throughout to monitor for hypertension caused by hypercalcaemia, or hypotension due to the anaesthesia.
With the animal in dorsal recumbency, a ventral midline incision was made on the cervical neck from the caudal larynx to the cranioventral thorax.
Blunt dissection through the sternohyoid and sternothyroid muscles exposed the trachea and larynx. Four parathyroid glands exist – associated with each thyroid is an external parathyroid, so called as it lies external to the thyroid capsule, and an internal thyroid that resides within the capsule.
The thyroid glands were isolated and, from there, the external and internal parathyroids were identified and examined. The right external parathyroid and left internal parathyroid were grossly enlarged, measuring approximately 4mm each.
Use of fine iris scissors and blunt dissection allowed removal of the right external parathyroid gland without damage to the thyroid. If an internal parathyroid gland is enlarged, then Fossum (2002) recommends removal of the entire thyroid.
It was found that by using a similar technique as was used with the external parathyroid, the internal gland was able to be removed intact without having to remove the thyroid. Wound closure and anaesthetic recovery were uneventful, and no bradycardias or other dysrhythmias were encountered.
Atrophy of non-adenomatous parathyroid glands can cause a transient hypocalcaemia following removal of affected glands. Therefore, serum calcium levels were checked three hours post-surgery and were 2.79mmol/L (1.97 to 2.99). Phosphorus was 1.36mmol/L (0.80 to 1.60).
If postoperative hypocalcaemia does occur, 10% calcium gluconate at 0.5ml/kg to 1.5ml/kg can be administered slow IV. This can be continued by adding 10ml 10% calcium gluconate to 250ml isotonic crystalloid (for example, saline or Hartmann’s solution) and administering at maintenance rate (2ml/kg/hr). Oral calcium and vitamin D can be continued at home if hypocalcaemia persists, although this is less likely if at least one parathyroid has been preserved.
Three days post-surgery, calcium levels had come down even further to 2.27mmol/L (1.97 to 2.99). The excised parathyroid tissue was sent for histopathology. Both glands were confirmed as parathyroid adenomas and confirmed to have been completely excised. The owner reported the polydipsia had improved dramatically since the surgery.
Primary hyperparathyroidism is a rare disease in dogs, but an important differential for hypercalcaemia. Presenting clinical signs are related to persistent hypercalcaemia, but may be limited to polyuria and polydipsia. Diagnosis of urinary tract infection should not prevent further investigation of hypercalcaemia, as up to 30% of dogs with hyperparathyroidism have concurrent UTI (Feldman et al, 2005).
Approximately a third of dogs with hypercalcaemia will not receive a diagnosis. Of dogs with a pathological cause for hypercalcaemia, approximately two-thirds will be due to neoplasia. The majority of these will be lymphoma. The next most common cause of hypercalcaemia is renal failure, followed by hyperparathyroidism (Messinger et al, 2009).
High calcium with an inappropriately high PTH gives a high probability of primary hyperparathyroidism (in the absence of evidence for secondary renal hyperparathyroidism). This can be confirmed with cervical ultrasonography, but may require specialist imaging technicians.
The treatment of choice is surgical removal of adenomatous parathyroid glands. This can be performed in the first-opinion setting – especially if one has experience with thyroid surgery and anatomy. Postoperative monitoring of hypocalcaemia is important until remaining parathyroid glands begin to produce PTH.
Key points that can be taken from cases such as this include: