17 Jul 2023
Lotfi El Bahri DVM, MSc, PhD discusses the implications a particular human laxative can have on dogs, using the case of a young female boxer.

Image: © Life in Pixels / Adobe Stock
You are presented at your emergency veterinary hospital with a female boxer aged seven months old, weighing 20kg.
The owner says he was faced with a lack of defecation from his dog for a few days and that he tried to give it an orally over-the-counter laxative a few hours before. The owner has noted lethargy, vomiting, ataxia and muscle tremors.
The bottle the owner brings with him shows that the active ingredient is a mixture of monobasic sodium phosphate 48g with sodium phosphate dibasic 18g in water, per 100ml delivered dose.
Monobasic sodium phosphate (also known as monosodium phosphate and sodium dihydrogen phosphate; NaH2PO4) and sodium phosphate dibasic (also known by disodium phosphate or disodium hydrogen phosphate; Na2HPO4) are salts of phosphoric acid (H3PO4). Sodium phosphates are administered by oral route as a saline laxative for the treatment of constipation and as a purgative for bowel cleansing prior to a colonoscopy in humans.
The cathartic action of sodium phosphate products results largely from its osmotic properties, drawing plasma water into the lower bowel, resulting in a build-up of pressure that triggers peristalsis and allows defecation to take place rapidly.
The oral median lethal doses of monobasic sodium phosphate and sodium phosphate dibasic are 8,290mg/kg and 5,950mg/kg, respectively, in rats. Poisoning in dogs can occur after administration of sodium phosphate by oral or rectal route (enema). In pets, sodium phosphate causes life-threatening metabolic disorders and can be deadly toxic.
Young animals appear to be more susceptible than adults. Studies suggest that the Staffordshire bull terrier may be a breed that is predisposed to hypernatraemia.
In humans, the US Food and Drug Administration has become aware of reports of severe dehydration and changes in the levels of serum electrolytes from people having taken more than the recommended dose of over-the-counter sodium phosphate products, resulting in serious adverse effects on organs such as the kidneys and heart – and in some cases, resulting in death. Health care professionals need to use caution when recommending an oral dose of these products for children five years and younger.
Sodium phosphate exerts toxic effects through several mechanisms.
Sodium is the dominant cation in extracellular fluid and necessary for the maintenance of intravascular volume. It is responsible for maintaining plasma osmolality between fluid compartments, and regulating water movement between the intracellular and extracellular compartments. Serum sodium is maintained within a narrow range (140mEq/L to 155mEq/L) in healthy dogs.
Excessive absorption of sodium phosphate products causes shift of fluid from the large intracellular fluid compartment to the extracellular fluid compartment. Massive water loss results in hypernatraemia and hyperosmolality. Hypernatraemia is caused by free water deficit (FWD). Each litre of free water lost increases serum sodium concentration by 3mEq/L to 5mEq/L. Additionally, water losses induce serum electrolyte disturbances.
Sodium phosphate, as a small hydrophilic molecule, is absorbed principally in the jejunum and duodenum by both passive diffusion and active transport. Absorption occurs passively along concentration gradients through tight junction complexes (claudins, occludins and junction adhesion molecule A) between cell membranes.
This pathway is non-saturable and constitutes 65% to 85% of intestinal phosphate absorption. Active transport is saturable and sodium-dependent. It occurs primarily through the sodium-phosphate cotransporter 2B (NaPi-2b), and, to a lesser extent, type 3 transporters (PiT-1 and PiT-2). This route constitutes about 15% to 35% of intestinal phosphate absorption. The absolute oral bioavailability of sodium phosphate is 60%.
An acute increase in phosphate load and a shift of fluid from the large intracellular fluid compartment to the extracellular fluid compartment produces massive tissue breakdown and cellular shifts of phosphate out the cells, resulting in hyperphosphataemia. Rapid increases in serum phosphate result in changes in calcium and magnesium, with clinical consequences. As calcium is necessary for normal physiological function, hypocalcaemia may especially affect the neuromuscular system with muscle stiffness or weakness.
Excessive free water loss produces severe hypovolaemia, decreased urine output and increased blood urea nitrogen (BUN). Blood pressure is often increased, due to expansion of the extracellular fluid compartment. Severe dehydration may also occur.
Excessive absorption of sodium phosphate can lead to other electrolyte disorders, including hypokalaemia due to intestinal potassium loss and hypomagnesaemia.
More than 95% of total body potassium (K+) is intracellular, where it is the most abundant cation. Potassium plays an important role in maintaining cell volume, enzyme function, protein synthesis, cell growth and neuromuscular activity. As serum K+ decreases, changes in cell excitability result in muscle weakness. The presence of hypokalaemia should alert the clinician to the possibility of coexisting hypomagnesaemia.
Magnesium, the second most abundant intracellular cation, regulates the movement of calcium into smooth muscle cells to maintain cardiac contractile strength.
The brain is the major organ affected by hypernatraemia. Severe hypernatraemia – and, therefore, hyperosmolality – lead to a shift of free water from intracellular space to extracellular space. This sudden shift is the cause of brain shrinkage and traction on the vasculature of the CNS, vascular rupture with cerebral bleeding, subarachnoid, subdural haemorrhage, increased intracranial pressure, central pontine and extra pontine myelinolysis and neurological injury.
Hypernatraemia and hyperosmolality has negative chronotropic (heart rate and rhythm) and inotropic (force of contraction of cardiac muscle) effects.
Clinical manifestations are usually seen when the serum sodium concentration is greater than 170mEq/L. They include the following.
CNS signs include:
Renal signs include:
Gastrointestinal (GI) signs include:
Cardiac signs include:
Laboratory values indicate:
ECG changes are observed. Hypernatraemia causes low voltage, QT interval prolongation (normal QT criteria in dogs is 0.15 to 0.25 seconds) and T-wave flattening. Prolongation of the QT interval is associated with high risk of ventricular arrhythmias and sudden cardiac death.
Sodium phosphate poisoning in dogs is a potentially life-threatening electrolyte disorder. Hypernatraemia should be approached with the same degree of urgency that we would use when treating hyperkalaemia. Veterinarians should be aware of the high toxicity of sodium phosphate products.
The patient should be transferred to an intensive care unit. No specific antidote exists. Treatment is aggressive, supportive care (replacement of free water loss, adequate correction of the electrolyte disorders, support of cardiovascular function).
Attention to airway and breathing is paramount. Intubate affected animals and provide artificial respiration with oxygen during convulsions (8L/mn to 12L/mn oxygen concentrations of 50% to 60%). Monitor ECG of the patient.
Convulsions should be controlled and may require attention for more than 24 hours. An IV catheter should be placed.
To control seizures, diazepam (0.5mg/kg to 2mg/kg IV bolus) should be administered and repeated if necessary within 20 minutes (serum half-life in dogs is 2.5 hours to 3.2 hours) up to three times in a 24-hour-period, or 1mg/kg to 2mg/kg rectally.
Do not give diazepam by IM. This is contraindicated in patients with severe liver disease, however.
Alternatively, administer lorazepam (long-acting benzodiazepine 0.2mg/kg IV bolus) because of its high affinity for benzodiazepine receptors in the CNS, or midazolam 0.2mg/kg to 0.4mg/kg IV may be repeated once.
When IV access is not available, midazolam can be administered by IM because it is rapidly absorbed by this route.
Ketamine used alone causes muscle rigidity and could potentially exacerbate seizures and tachyarrhythmia, while valproic acid is not recommended (serum half‑life in dogs is between 1.5 hours and 2 hours).
If seizures persist or recur, administer propofol (3mg/kg to 6mg/kg IV initial bolus) followed by 0.1/kg/min to 0.6 mg/kg/min constant rate infusion (CRI).
Alternatively, 2% to 2.5% concentrations of isoflurane alone with oxygen can also be used.
For maintenance, use 1.5% to 1.8% concentrations of isoflurane in oxygen.
The aim of treatment for severe hypernatraemia is to correct volume circulatory, replace free water losses, and replace ongoing losses and maintenance fluid.
In the hypovolaemic/hypernatraemic patient, correct volume circulatory by administration of sterile isotonic crystalloids before efforts to correct hypernatraemia take place.
The initial replacement fluid of choice is IV isotonic saline sodium chloride 0.9% (normal saline). This solution has 154mEq of sodium per litre and is relatively hypotonic in hypernatraemic patients.
The crystalloid dosage for severe hypovolaemia in dogs is 80ml/kg/hour to 90ml/kg/hour IV. One-quarter to one-third of the dose should be given across 15 to 20 minutes, followed by reassessment of the patient’s vital signs, including mucous membrane colour, capillary refill time, heart rate and blood pressure.
Fluids administered subcutaneously are not absorbed well because blood flow is diverted to the heart, lungs and brain during a hypovolaemic state. The use of synthetic colloids (for example, hydroxyethyl starch) is relatively contraindicated in the case of severe hypovolaemia/hypernatraemia.
The cornerstone of hypernatraemia treatment is also to replace FWD, which reduces the serum sodium to a level that results in no clinical signs associated with hypernatraemia.
Once IV volume depletion has been restored, replace the FWD by administration of isotonic 5% dextrose (D-glucose) in water (D5W). Giving D5W is equivalent to giving free water, because glucose is rapidly metabolised by cells, leaving free water for use by the body.
The volume of FWD in litres that must be replaced, is calculated using the following equation:
FWD (L) = 0.6 × bodyweight (kg) × ([serum Na+ patient/normal serum Na+] – 1)
Half of the water deficit should be replaced over the first 24 hours, and the remainder replaced over the following 24 to 72 hours, with the total correction taking place over 48 to 96 hours by 5% dextrose.
When hypernatraemia develops acutely, the correction can occur rapidly, with the FWD being replaced to achieve a rate of change of serum sodium by a maximum of 0.5mEq/L to 1.0mEq/L per hour in the first 24 hours to avoid rapid brain cell swelling, cerebral oedema and severe neurologic damage, followed by correction gradually within the next 48 hours.
Monitoring of the serum sodium concentration every 4 to 8 hours for the first 24 hours is necessary to ensure that it is not decreased too quickly and to adjust the FWD appropriately. Drinking can be encouraged by placing flavouring in the water (beef or chicken stock mixed, and made into ice cubes and added to the water).
Infusing D5W may be challenging in diabetic patients because hyperglycaemia itself leads to poor outcomes in critically ill patients. An increase in insulin dosing may be required.
Significant GI losses from diarrhoea and vomiting are calculated based on a predicted fluid amount lost by a patient within a 24-hour period. Current recommendations are to deliver 2ml/kg/hour of lactated Ringer’s solution (LRS; 130mEq/L Na+) with 10mmol/L to 20mmol/L potassium chloride added if required.
Cells have a daily water requirement to maintain regular metabolism. The maintenance fluid requirement of LRS over 24 hours is calculated using the following formula:
132 × (bodyweight)0.75 per 24 hours
The rule of thumb is 2ml/kg/hour to 6ml/kg/hour.
Regarding treatment of hyperphosphataemia, administer diuretics such as acetazolamide – a potent inhibitor of the enzyme carbonic anhydrase – that works on the proximal tubules to promote renal phosphate excretion: 50mg/kg IV one time.
Reconstitute 500mg vial with at least 5ml of sterile water for injection; use within 24 hours after reconstitution. Acetazolamide is associated with relatively potential adverse effects (for example, GI disturbances, CNS effects).
Regarding treatment of hypocalcaemia, administer calcium gluconate 10% solution (warmed to a body temperature) 0.5ml/kg to 1.5ml/kg IV slowly over a 20 to 30-minute period, with ECG monitoring. If bradycardia develops – which signals the onset of cardiotoxicity from an excessively rapid infusion rate of calcium – halt infusion.
Calcium gluconate is often chosen because it is non-irritating if the solution is injected outside of a vessel. In contrast, calcium chloride (0.15ml/kg to 0.5ml/kg IV slowly) is extremely irritating to tissues, but provides more elemental calcium in each millilitre of solution.
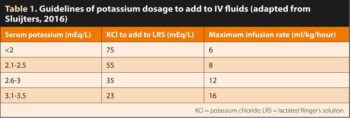
The immediate goal of treating hypokalaemia (raising serum potassium to a safe level) is the prevention of potentially life-threatening cardiac conduction disturbances and neuromuscular dysfunction. Potassium is added as potassium chloride (KCl) and the dosage depends on the dog’s serum potassium levels (Table 1).
Because the use of IV potassium increases the risk of hyperkalaemia and can cause pain and phlebitis, IV potassium should be reserved for patients with severe hypokalaemia. Administration of fluids with greater than 60mEq/L of potassium through peripheral catheters is not recommended because phlebitis can result.
Potassium-containing fluids should never be used as a bolus or delivered at high rate of concentration. Do not exceed an IV administration rate of 0.5mmol/kg/hour of potassium as toxicity and death may occur. Where possible, it is recommended to use infusion pumps to ensure accurate administration rates.
It is essential to mix added KCl thoroughly in the IV bag, as inadvertent potassium overdoses can occur and are often fatal.
Concomitant hypomagnesaemia aggravates hypokalaemia and renders it refractory to treatment by potassium.
The treatment of hypomagnesaemia is determined by its severity and the patient’s clinical status. IM administration of magnesium sulphate is painful, and should be reserved for patients with severe hypomagnesaemia and limited venous access. For severe hypomagnesaemia, administer magnesium sulphate 20% solution in a 0.9% sodium chloride at a CRI of 1.6mg/kg/hour to 2.5mg/kg/hour.
IV infusion is the preferred means of delivering fluids to severely dehydrated animals. Fluid therapy with IV sterile sodium chloride 0.9% should always be the first-line management.
The volume (ml) of fluid needed to correct dehydration is calculated from the following formula:
Percentage of dehydration × bodyweight (kg) × 1,000
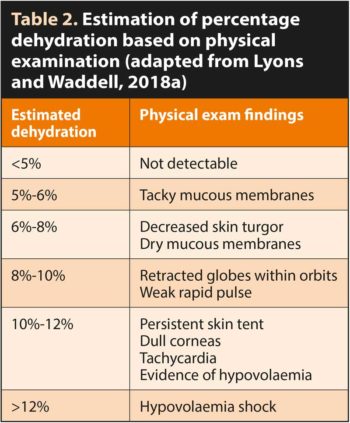
Percentage dehydration can be estimated by examining mucous membrane moisture, skin turgor, eye position and corneal moisture, as described in Table 2.
It is recommended that one-fourth to one-half of the estimated dehydration deficit be replaced over the first two to four hours, with the remaining dehydration deficit and maintenance isotonic volumes administered over the subsequent 20 to 22-hour period. Adequate hydration and urine output (1ml/kg/hour to 2ml/kg/hour) should be ensured.
To treat severe hypotension, administer dopamine – an endogenous catecholamine vasopressor, which stimulates cardiac beta-1 adrenergic receptors by releasing norepinephrine, and increasing heart rate and contractility. The dose is 5µg/kg per minute to 20µg/kg per minute CRI.
To treat metabolic acidosis, administer buffer therapy to correct the metabolic acidosis (normal values in dogs: pH lower than 7.33; standardised base excess lower than -4mmol/L). Administration of sodium bicarbonate is contraindicated, since the sodium will exacerbate the hypernatraemia, worsen hyperosmolality and also decrease ionised calcium, and may expose clinical signs of hypocalcaemia.
Administer tris(hydroxymethyl)aminomethane (tromethamine; THAM) – a weak base amino-alcohol with amine groups, which actively binds hydrogen ions to correct acidaemia. THAM has a greater buffering capacity than bicarbonate (acid dissociation constant of 8.07 versus 6.4, respectively) and is effective in buffering both metabolic and respiratory acidosis, which leads to an increase in intracellular and extracellular pH.
In humans, THAM solution (3.6g per 100ml) is administered with a loading dose of 2mmol/kg to 4mmol/kg over 20 minutes, followed by a CRI of 0.5mmol/kg/hour to 1.0mmol/kg/hour for 4 to 10 hours.
Activated charcoal is contraindicated since it doesn’t bind the mineral toxicants and can potentially contribute to hypernatraemia.
Charcoal pulls fluid from the body into the small intestine, resulting in an elevation of the serum sodium concentration.
Owners should be advised to avoid foods that are high in phosphates: pork (28mg/100g), chicken and turkey (23mg/100g), liver (18mg/100g), cheese, beans (176mg/100g) and lentils (36mg/100g). However, adequate protein intake should not be compromised in the restriction of dietary phosphate. In critically ill patients, protein requirements are 1.5g/kg/day to 2g/kg/day. Patients can be discharged only with normal biochemical parameters and no neurological sequelae.