6 Mar 2023
Laura Bree DipECVIM, MVB, MRCV and Jessica Dean BVetMed, CertAVP(SAM), MRCVS provide a comprehensive outline for diagnosing and monitoring these endocrinopathies in dogs.

Figure 2. Calcium homeostasis (Song, 2017).
Canine hypothyroidism is a common endocrinopathy in dogs. It can be easily misdiagnosed due to non-thyroidal illness, as well as the difficulties in interpreting results of diagnostic testing. Clinical signs and biochemical changes are often vague and non-specific.
When achieving a diagnosis of canine hypothyroidism, clinicians must assess the dog’s history, clinical signs, physical examination findings, clinicopathological changes and results of thyroid hormone testing in tandem.
Each canine thyroid gland consists of two lobes and is situated either side of the trachea, and has a primary purpose of producing active thyroid hormones, predominantly thyroxine (T4) and triiodothyronine (T3).
Almost all thyroid hormones are protein bound, acting as a reserve of thyroid hormones for delivery to tissues, while unbound “free” thyroid hormone is metabolically active.
The majority of free T3 (fT3) is not produced within the thyroid gland itself, but produced by systemic modification of T4 to T3. Free T3 is approximately five times more potent than free T4 (fT4).
Thyroid hormone affects a majority of metabolic processes throughout the body, including metabolic rate, enzyme and protein synthesis, as well as carbohydrate and lipid metabolism. Thyroid hormone, therefore, influences – for example, but not limited to – growth, erythropoiesis, and cardiovascular and CNS development.
Regulation of thyroid hormone synthesis is tightly controlled. The hypothalamus releases thyrotropin-releasing hormone (TRH) in response to reducing concentrations of fT4 and fT3.
The pituitary gland is stimulated by TRH to release thyroid stimulating hormone (TSH), which stimulates the thyroid gland to produce T4 and T3.
Inhibitory feedback to the hypothalamus and pituitary gland is achieved by increase in fT4 and fT3. Glucocorticoids and TSH also provide negative feedback on the production of TRH.
Medications have various effects on thyroid hormone synthesis and homeostasis.
Hypothyroidism can be primary (lack of T4 and T3 production) or central (TSH deficiency), and is either congenital or acquired. Primary acquired hypothyroidism is by far the most commonly identified form of canine hypothyroidism.
Congenital hypothyroidism is significantly more rare than the acquired form and is generally due to central hypothyroidism. It results from hypoplasia, aplasia, dysgenesis or dyshormonogenesis (Mooney and Shiel, 2012). In some cases, this causes disproportionate dwarfism.
Central hypothyroidism results from deficient TSH secretion from the pituitary gland, which results in atrophic degeneration of the thyroid gland (Mooney and Shiel, 2012).
TSH deficiency has been reported in dogs with pituitary dwarfism and dogs with thyroid dysgenesis. The miniature schnauzer has recently been identified as a breed affected by central hypothyroidism (Voorbij et al, 2016).
Where causative mutations for congenital hypothyroidism have been identified, such as the toy fox terrier, genetic testing is available. In the toy fox terrier, an autosomal recessive mutation results in dyshormonogenesis and thyroid peroxidase deficiency, causing primary congenital hypothyroidism and a goitre (Fyfe et al, 2003).
Mutations causing a similar phenotype have also been reported in the Tenterfield terrier, Spanish water dog and papillon. Congenital hypothyroidism could also develop secondary to puppies born to iodine deficient dams or those fed either a restricted iodine diet, or one that is in excess from birth.
Acquired primary hypothyroidism accounts for the majority of hypothyroid dogs.
A sensitive test is likely to be positive in a positive case; the higher the sensitivity, the less likely a false negative is incurred.
A specific test is likely to be negative in a negative case; the higher the specificity, the less likely a false positive is incurred.
A good example is thyroid stimulating hormone (TSH), where the sensitivity is reduced and, therefore, it is possible to have a false negative (that is, a normal) TSH concentration in a hypothyroid dog. However, its high specificity means that if TSH concentration increased, a very low chance exists this is a falsely positive result.
It is caused by lymphocytic thyroiditis and thyroid follicular atrophy, with authors theorising that follicular atrophy may be the end-stage consequence of lymphocytic thyroiditis, rather than a form of idiopathic atrophy.
Histologically, lymphocytic thyroiditis is inflammatory, with marked infiltration of lymphoplasmacytic cells and macrophages. The initiator of thyroid inflammation is still not fully understood, although it is thought genetics play a role. This has been demonstrated in the different rates of progression in different breeds. Surgical removal of thyroid glands will result in primary hypothyroidism.
Histopathological diagnosis of primary hypothyroidism does not have an effect on diagnosis or therapy of the disease, and is uncommon. However, it is important to be aware that the stage of lymphocytic thyroiditis will have an effect on results of thyroid testing – that is, whether the dog is early or later into the disease process.
Four different stages of lymphocytic thyroiditis have been suggested, with each stage affecting total T4 (TT4), TSH and thyroglobulin autoantibody (TgAA) concentrations. For example, in early disease, we may identify increased TgAA concentrations only (silent thyroiditis), with that dog later developing low circulating TT4 and increased TSH concentrations.
Later on in the disease, TgAA is rarely increased and TSH concentrations are actually low.
Pituitary neoplasia or surgical hypophysectomy would account for the majority of reported acquired central hypothyroidism, but this would still only account for less than 5% of hypothyroid dogs.
Hypothyroidism is most often seen in middle-aged to older dogs. Dogs of any sex and neutered status can be affected, with a slightly higher risk within neutered females. Breeds most commonly presented include – but are not confined to – the English setter, golden retriever, Rhodesian ridgeback, cocker spaniel and boxer.
Historical findings are often vague, but owners generally observe lethargy, poor exercise tolerance, weight gain, intolerance to cold and mental dullness.
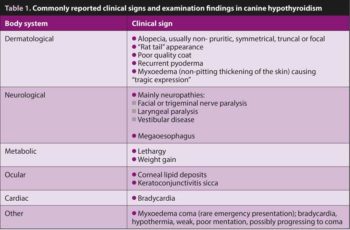
The most common clinical signs are metabolic and dermatological. Less commonly, neurological deficits are noted; however, the relationship between hypothyroidism and neurological signs is not fully confirmed, and neurological signs alone are rarely an indication to consider primary hypothyroidism. Table 1 describes commonly reported clinical signs and examination findings.
Changes seen in routine haematology and biochemistry are non-specific, mild and can vary between individuals. Haematology can detect a mild normochromic, normocytic anaemia in up to 40% of cases, with haematocrit levels rarely below 25%. Hypothyroidism has been associated with increased platelet counts with smaller platelets. Some dogs are reported to easily bruise; this is thought to be due to decreased plasma von Willebrand factor antigen concentration. Most commonly reported biochemical changes include hypercholestrolaemia (75% of dogs), often with hypertriglyceridaemia, mild increase in alkaline phosphatase activities (30% of dogs), as well as mild increase in gamma-glutamyl transferase activity.
Recently, it was shown that mean symmetric dimethylarginine (SDMA) and creatinine concentrations were higher in hypothyroid dogs when compared to euthyroid dogs; however, SDMA was rarely above the reference interval (less than 18ug/dL; Di Paola et al, 2021). In the majority of these dogs, the creatinine normalised following levothyroxine supplementation.
TT4 and TSH concentrations should be measured as part of a minimum data base in the investigation of hypothyroidism. The sensitivity of T4 is good; therefore, dogs with a T4 concentration within the reference range should be considered unlikely to have hypothyroidism.
Keep in mind that T4 assays may interpret TgAA as T4, presenting a falsely normal T4 concentration. In young dogs with potential early disease, TgAA should be measured in tandem to avoid missing an early diagnosis. By contrast, a low T4 concentration alone is commonly caused by non-thyroidal illness and, therefore, has a low specificity for hypothyroidism.
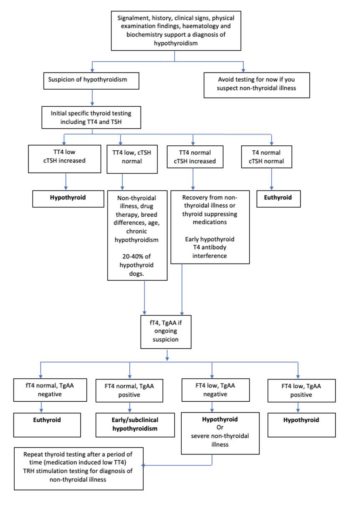
As previously mentioned, TSH concentrations are typically increased at the time of diagnosis in hypothyroid dogs; however, TSH concentrations can be low or normal in chronic hypothyroidism due to the exhaustion of the pituitary gland, or due to daily variation, effects of drugs and non-thyroidal illness.
Dynamic/stimulated thyroid function testing (TSH/TRH stimulation) is uncommonly performed and reserved for cases where baseline function testing is unable to achieve a diagnosis. TSH stimulation was once considered the gold standard for diagnosis.
Whereby a euthyroid dog would increase its basal thyroid concentration by 1.5 fold, or an absolute value of more than 30nmol/L, and hypothyroid dogs would fail to increase T4 or have an absolute value of less than 20nmol/L. Many cases were noted to be between cut-offs, making the test less useful.
Bovine TSH was classically used; now unavailable, more expensive recombinant human forms of TSH are available. Due to cost and also the potential for equivocal results, TSH stimulation is not commonly used.
TRH stimulation testing was classically proven to be less reliable than standard thyroid basal testing; however, a recent study using recombinant human thyrotropin was able to successfully differentiate hypothyroid dogs and those with non-thyroidal illness (Corsini et al, 2021).
A post-stimulation T4 concentration of more than 21nmol/L was indicative of normal thyroid function (sensitivity 100%, specificity 93%). For the time being, TRH stimulation testing is mostly used in research studies.
Table 2 illustrates commonly used thyroid tests by their limitation, sensitivity and specificity. See Figure 1 for a flow chart for a logical clinical pathway for diagnosis of hypothyroidism.
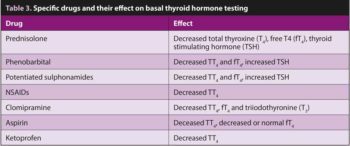
Clinicians should always document concurrent drug therapy before investigating hypothyroidism. Medications and their proposed effects on thyroid hormones are described in Table 3.
Recently, toceranib – a tyrosine kinase inhibitor – has been shown to increase TSH concentrations, but when used in the short term, has not been associated with the development of decreased T4 concentrations and hypothyroidism (Harper et al, 2020).
Increasing age has been shown to be associated with a decreasing TT4 and fT4 concentration, alongside an increase in TSH concentration, albeit minor (Hegstad-Davies et al, 2015).
A large variation in T4, fT4 and TSH has been identified between different purebred dogs (Hegstad-Davies et al, 2015), with a significant increase in TT4 and fT4 concentrations also identified in females.
Euthyroid greyhounds will often have TT4 concentration below the lower limit of the non-breed reference interval; TT3 measurement is useful for diagnosis (Shiel et al, 2007).
Approximately 30% of sick dogs will have a T4 below the standard reference range (Mooney et al, 2008). TSH is more often normal in these dogs, and fT4 is less likely to be reduced.
It is thought that the magnitude of the decrease of T4 in non-thyroidal illness is related to severity of disease and could be used prognostically (Nishii et al, 2019). It is advised to not investigate hypothyroidism in unwell animals; however, if a very strong clinical suspicion exists, testing should include TT4, fT4 and TSH.
This is due to the fact that it is not common for concurrent non-thyroidal illness to cause a low TT4, low fT4 and high TSH.
Some evidence exists that secretion of TSH from the pituitary gland can decrease with hypothyroidism. This is supported by a small study documenting TSH concentrations within the reference interval in some hypothyroid dogs (Diaz-Espineira et al, 2008).
Levothyroxine therapy affects the hypothalamic-pituitary-thyroid-axis, making interpretation of thyroid testing for hypothyroidism inaccurate while on therapy.
A recent study has demonstrated normal thyroid function (TT4, fT4 or TSH) in euthyroid patients within one week of stopping levothyroxine therapy (Ziglioli et al, 2017).
Where misdiagnosis is ever suspected, the drug should be withdrawn and re-testing can occur after one week, without serious side effects for the dog.
Dogs with hypothyroidism require chronic hormone replacement therapy.
The prognosis for treated hypothyroidism is excellent. Synthetic sodium levothyroxine therapy is the treatment of choice. This contains T4 alone, and several available licensed formulations in veterinary medicine exist.
Combination products for human use exist, containing T3 and T4, but these are not recommended, as they do not reflect the normal hormone ratio in dogs.
The ideal dosing regime is generally agreed to be 20μg/kg to 22μg/kg by mouth every 24 hours (Dixon et al, 2002). The response to once daily dosing is excellent. Improvements in cardiovascular function and, for example, ECG abnormalities are usually seen within the first eight weeks.
Weight loss is a common response to initiation of therapy, but often limited to a 10% weight loss within the first three months.
Improvement in dermatological changes are seen within the first month and continue over time. Improvement in the neurological system is more variable and can take around six months to become apparent (Utsugi et al, 2014).
Evidence is limited on improvement of megaoesophagus or laryngeal paralysis in affected dogs, but this is likely due to an unclear relationship between hypothyroidism and these conditions (Mooney et al, 2012).
Monitoring is based on the combination of improvement of associated clinical signs and peak TT4 concentrations. Peak TT4 concentrations will occur four to six hours after once daily dosing. Laboratory monitoring can start following two weeks of therapy.
Monitoring is recommended every six months following determination of the ideal dose. Concurrent measurement of haematology and biochemistry annually to biannually is considered good practice.
Measuring TT4 and TSH in tandem is considered best practice to fully assess control of hypothyroidism.
Keep in mind that measurement of TSH is only useful in those dogs where it was increased prior to starting thyroid hormone replacement therapy.
Canine primary hypoparathyroidism (PHP) is an uncommon endocrinopathy of dogs that results in deficiency of parathyroid hormone (PTH), leading to serum hypocalcaemia and serum hyperphosphataemia.
Due to the low incidence of primary hypoparathyroidism, clinicopathological and diagnosis data on the disease in dogs is limited to approximately 45 cases across the past 50 years.
Much of what we know about the condition originates from these cases, as well as human medicine, where the aetiology is believed to be similar.
Four parathyroid glands (two glands associated with each thyroid lobe) exist that release PTH hormone in response to small changes in serum ionised calcium.
Negative feedback and inhibition of PTH release relies on ionised calcium and vitamin D concentrations. Positive feedback begins with a decrease in serum ionised calcium. This leads to immediate PTH secretion, rapidly inducing calcium release from bone, increasing calcium absorption and phosphorus excretion by the distal tubule of the kidney, and simultaneously stimulating 1-alpha-hydroxylase activity in the renal tubules, causing an increase in synthesis of vitamin D. Vitamin D acts on the gastrointestinal (GI) tract to increase calcium and phosphorus absorption. Loss of PTH activity leads to serum hypocalcaemia, which cannot be compensated (Figure 2).
Calcium is tightly regulated to closely maintain narrow reference intervals. Total serum calcium concentrations are usually 2.3mmol/L to 2.8mmol/L in dogs. Complete assessment of calcium should include ionised calcium measurement – about 50% of total body calcium is ionised, 40% is bound to protein, mainly albumin, and approximately 10% is complexed with anions.
When exploring causes of hypocalcaemia or, indeed, hypercalcaemia, it is important to remember this distribution; for example, hypoproteinaemia will induce total hypocalcaemia, but should not affect ionised calcium concentration.
A lack of PTH leads to decreased total and ionised calcium, where the biologically active ionised calcium is more useful to assess severity of hypocalcaemia.
Other causes of hypocalcaemia can be due to chelation, saponification or sequestration of the calcium in the body due to various diseases, toxins or physiological states.
It is debated, but generally accepted, that canine PHP results from immune-mediated destruction of the parathyroid glands. Histopathological examination of a small number of cases in the literature was consistent with lymphocytic plasmacytic parathyroiditis, similar to humans (Bruyette and Feldman, 1988). Other causes are generally acquired, such as surgical removal of the parathyroid glands. In humans, it is also reported as part of polyendocrine syndromes and can rarely be idiopathic.
While history, signalment, examination, baseline blood work and the severity of ionised hypocalcaemia can produce a very high index of suspicion for PHP, other causes of hypocalcaemia should be considered before making a diagnosis (Figure 3).

A small portion of dogs presenting with PHP do so in emergency, with signs of clinical tetany or seizures. Immediate therapy with injectable calcium gluconate is generally required before a full assessment is possible.
Once possible, a thorough history is necessary to identify any pertinent clues for causes of hypocalcaemia, such as recent polydipsia, polyuria, GI signs, abdominal discomfort, weight loss or exposure to toxins, medications, pregnancy, and to obtain detail on the dog’s diet. Examination should involve careful attention to signs of chronic disease, such as cachexia and softening of the mandibular bones, as well as acute disease, such as dehydration, abdominal pain or neurological signs.
Minimum database blood work identifies hypocalcaemia as a cause of the dog’s tetany and helps exclude many differential diagnoses; for example, renal disease and typical Addison’s disease can be excluded based on otherwise normal electrolytes and normal renal parameters.
Although PHP is rare, certain breeds appear over-represented in the available literature, including the St Bernard, poodle, Labrador, Scottish terrier and miniature schnauzer. Males and females appear to be similarly affected, and the age of presentation varies from six months to 12 years.
The majority of cases present to their primary care practices within one week of observing clinical signs, others present acutely, while some owners report more than four weeks of mild signs at home prior to an acute presentation.
The most common clinical observations include tetany, gait abnormalities, seizures, weakness, agitation, inappetence, vomiting, panting and facial rubbing (Russell et al, 2006).
Less common include aggression and cataracts. Clinician awareness of “latent tetany” is important: latent tetany occurs during a prolonged progressive decline in calcium concentrations, leading to a physiological adaptation. This can clinically be represented by a patient with relatively mild signs of hypocalcaemia despite an extremely low calcium concentration; for example, a patient with PHP may present with mild noise or physically induced tetany, but is ambulatory and relatively bright.
Total and ionised hypocalcaemia, with hyperphosphataemia (can be within reference range, commonly above) and normal renal parameters, are the hallmarks of PHP on biochemical analysis.
No gold standard diagnosis exists; however, exclusion of other causes of hypocalcaemia, low or normal PTH concentration, alongside ionised hypocalcaemia, is considered sufficient for a diagnosis of PHP.
Many authors argue the measurement of PTH (which is costly and takes up to two weeks) is academic in patients where other causes of a persistent ionised hypocalcaemia of less than 1.2mmol/L have been excluded. Vitamin D measurement should be performed in patients with concurrent granulomatous disease, such as idiopathic panniculitis, or those with unknown dietary history, to exclude a lack of vitamin D production, release or action.
To exclude pancreatitis, a history, physical examination, canine-specific pancreatic lipase immunoreactivity test and abdominal ultrasound may be necessary in total or in part.
Initially, a tetanic dog should be given the IV 10% calcium gluconate at 0.5ml/kg to 1.5ml/kg (5mg/kg to 15mg/kg) slowly, over 10 to 15 minutes. Most clinicians will dilute this volume with sodium chloride (NaCL) to reduce the effect of tissue necrosis should extravasation occur.
An ECG should be applied prior to IV administration and maintained throughout to identify any bradycardia, premature ventricular contractions or shortening of Q-T intervals. The clinical stabilisation of the dog occurs faster than the normalisation of ionised calcium. Once the dog is stabilised, ionised calcium can be reassessed, and calcium gluconate should be added to any maintenance fluid (usually NaCL) and given at 2.5mg/kg/hour to 3.5mg/kg/hour (0.3ml/kg/hour to 0.4 ml/kg/hour; Galvão et al, 2017).
As an example: for a 10kg dog, initial stabilisation uses approximately 10ml of 10% calcium gluconate as a bolus over 15 minutes. Making up the dog’s calcium gluconate solution for CRI infusion requires careful calculation. It is generally safer to make up a simple solution of known calcium per millilitre (Ca/ml) concentration; for example, adding 50ml of calcium gluconate to 500ml of NaCL to make a solution of 0.93mg Ca/ml, where each millilitre of 10% calcium gluconate contains 9.3mg/ml of Ca/ml. The 10kg dog would then receive 26ml per hour to achieve a dose of 2.5mg/kg/hr of calcium gluconate.
Extreme care should be given to the IV catheter site, as extravasation leads to severe tissue necrosis. Repeated boluses of calcium gluconate are usually not indicated and only recommended for signs of tetany.
Calcium (ionised) concentrations should be assessed once to twice a day, and the calcium infusion be adjusted accordingly.
Vitamin D analogues should be started in the first 48 hours, once other differentials have been excluded, to reduce hospitalisation time.
Oral calcium supplementation is favoured by some clinicians to speed up or enhance the effect of commenced vitamin D therapy, due to an inability of dogs to absorb calcium from bone, gut or kidney without PTH and/or vitamin D.
Others argue that a balanced diet and normal GI tract should be able to absorb sufficient calcium once vitamin D is commenced.
As calcium supplementation is relatively safe, it can be given for the first one to two weeks of therapy. The starting dose in dogs is 1g/day to 3g/day divided into two doses; calcium carbonate is most commonly used, where 1g of calcium carbonate contains 400mg of elemental calcium.
For our aforementioned 10kg dog, the authors would recommend giving 2g of calcium carbonate per day.
Calcium carbonate can be bought over the counter in most pharmacies, with the elemental calcium detailed on the data sheet to allow for accurate dosing.
Following oral supplementation of vitamin D and calcium, and stabilisation of ionised calcium concentrations (typically resting, hospitalised dogs become clinically stable close to 1mmol/L), the CRI of calcium gluconate can be weaned and the patient discharged 24 hours later.
Vitamin D analogues vary mostly by their preparation, time to onset, duration of action and potency due to the variation in their active analogue. Their use is most often dictated by what is available on the veterinary and human markets at the time of diagnosis, and the size of the dog.
The most commonly used analogues include calcitriol, alfacalcidol (1-alpha) and dihydrotachysterol (AT10), with other vitamin D analogues such as calcifediol and cholecalciferol less commonly used.
The authors favour calcitriol products overall due to its rapid onset of action compared to other analogues, but due to its formulation, it cannot be given safely to patients weighing less than 10kg, whereby the liquid formulation of 1-alpha is ideal for smaller doses.
The dose for each analogue is different and requires careful calculation based on the specific product preparation. It is best administered with food.
Following weaning of IV calcium, the dog should ideally remain in hospital for observation for a further 24 hours to assess for any decline in ionised calcium concentration. When the dog arrives home, becomes excited and exercises, a relapse of signs is often observed. The owner should be advised to keep the dog calm and resting for a further week while vitamin D therapy can establish normal total calcium homeostasis.
Prolonged time to vitamin D therapy response, as well as the rising costs of intensive care monitoring and hospitalisation, are major limitations to therapy with some dogs euthanised at the time of diagnosis due to financial restraint.
The long-term prognosis of PHP in dogs receiving therapy is good. Side effects of vitamin D therapy can be commonly observed in the first few months (signs of hypercalcaemia), while therapy is undergoing adjustment, and later in life as metabolism, body weight and vitamin D needs change.
This necessitates monthly monitoring of ionised and total calcium, phosphorus and renal parameters for the first months of therapy, followed by repeat assessments every three to six months, depending on the response to therapy and the dog’s life stage.
Dogs with PHP should be monitored for life, observing for chronic kidney disease and signs of mild chronic hypercalcaemia, due to the use of vitamin D therapy.