24 Jul 2017
Yordan Fernandez and James Warland look at multiple endocrinopathies in dogs, diagnosing the various disorders and the management options.

This patient had a combination of hyperadrenocorticism and diabetes mellitus, and showed marked polydipsia, a common clinical sign to both conditions, making clinical monitoring more challenging.
The presence of multiple endocrinopathies is uncommon in dogs, and the diagnosis and management of these patients has not been well described in veterinary literature. However, the recognition of concurrent endocrinopathies is important for the successful management of these cases. Often, the failure to recognise a second condition will lead to unsatisfactory outcomes, and frustration for vet and owner. The presence of multiple endocrine diseases can represent a diagnostic challenge because clinical signs and clinicopathological findings may be similar between diseases. Therefore, the diagnosis should be based on a combination of historical, physical, clinicopathologic and imaging findings.
Endocrine testing has some limitations given the presence of concurrent diseases and a careful interpretation of endocrine test results is extremely important, given the potential for false-positive results. The most common concurrent canine endocrinopathies include diabetes mellitus (DM) and hyperadrenocorticism, followed by hypoadrenocorticism and hypothyroidism; and hypothyroidism and DM. Appropriate treatment of the concurrent endocrine disorders is key to achieving a successful outcome for these patients.
The prevalence of multiple endocrine disorders in dogs in general practice is unknown.
In a study performed in a referral hospital, the prevalence of multiple endocrine disorders was low, representing 0.3% and 2.3% of the total canine population and total canine population with an endocrinopathy, respectively1. As diagnosis and management of canine endocrine disease improves, the recognised prevalence of concurrent endocrinopathies is likely to increase.
In human medicine, the presence of concurrent endocrinopathies is well described. These multiple endocrine disorders can be caused by multiple neoplasms of the endocrine glands (multiple endocrine neoplasia syndromes; MEN), or concurrent or progressive development of immune-mediated disease of separate endocrine glands, usually leading to hormonal deficiencies (known as autoimmune polyglandular syndromes)2.
In contrast to human medicine, little information exists regarding the diagnosis and treatment of dogs with multiple endocrine diseases in veterinary medicine. Moreover, although MEN and autoimmune polyendocrinopathy syndromes have been described in dogs, it seems dogs diagnosed with concurrent endocrinopathies are often unrelated to either MEN or polyglandular syndromes (for example, dogs with concurrent hyperadrenocorticism; HAC, and diabetes mellitus; DM)2.
The largest study to date of dogs with naturally occurring multiple endocrine disorders included only 35 dogs1. Based on this study, the most common concurrent canine endocrinopathies were DM and HAC, followed by hypoadrenocorticism and hypothyroidism, then hypothyroidism and DM. Concurrent endocrine diseases may be diagnosed at first presentation, further endocrinopathies may develop during the course of managing the initial condition, or later in life.
Breeds over-represented in dogs with multiple endocrine disorders include miniature schnauzers and poodles1,3. The age at the time of the diagnosis of the first endocrine disorder varies and depends on the endocrine disease (for example, young adult dogs for hypoadrenocorticism and middle-aged to older dogs for DM). Time variation is large between the diagnosis of the first and second endocrine disorder, with a median of four months and a range from 0 to 53 months in one study1. A clear female predisposition is not present in dogs with endocrinopathies as described in human medicine; the two largest studies – including dogs with multiple endocrinopathies – identified no gender predisposition1,3.
The diagnosis of concurrent endocrine diseases represents a diagnostic challenge because some clinical signs and clinicopathological findings may be similar between different endocrinopathies. Therefore, the diagnosis of concurrent endocrinopathies should be based on a combination signalment, clinical signs, physical examination findings and clinicopathological abnormalities, supported by specific endocrine testing. The presence of concurrent diseases makes interpretation of endocrine testing challenging given the potential for false-positive results.
The most common combination of endocrine diseases identified in veterinary literature is DM and HAC1,4. In the majority of dogs with both endocrine diseases, DM is diagnosed first and HAC is diagnosed either at the same time or at a later stage1,3. This does not necessarily mean DM occurs first, as this could be attributed to the ease to diagnose DM with routine investigations (for example, hyperglycaemia and glycosuria) and the similar clinicopathological signs seen in both diseases (for example, polyuria/polydipsia, polyphagia, increased alkaline phosphatase activity, diluted urine and proteinuria).
However, once DM becomes difficult to control, despite increasing dosages of insulin, then further investigations, including adrenal function testing, are often pursued. Therefore, it is important to consider the presence of concurrent HAC in diabetic patients with persistent or recurrent clinical signs, as HAC is one the most common causes of insulin resistance in diabetic patients4.

The suspicion of HAC should increase in a poorly controlled diabetic dog with clinical signs consistent with HAC, but not DM (for example, symmetrical alopecia and calcinosis cutis), and the lack of other reasons for insulin resistance, including inadequate handling of the insulin, oestrus or other concurrent diseases, such as urinary tract infections (UTIs) or chronic pancreatitis. Apart from the compatible clinicopathological and imaging findings (for example, bilateral adrenomegaly, the presence of an adrenal or pituitary mass), a diagnosis of HAC should be confirmed with the results on an adrenocorticotropic (ACTH) stimulation test or a low-dose dexamethasone suppression test (LDDST). However, the results of any endocrine tests should be interpreted cautiously because concurrent diseases, such as DM, can lead to false-positive adrenal function test results. In fact, the false-positive result rate of the LDDST and ACTH stimulation test can be as high as 30% and 15%, respectively, in dogs with concurrent diseases or receiving medications4.
Clinical suspicion is important in the interpretation of adrenal testing in any dog. However, given the increased risk of false-positive results for HAC in a patient with DM, or any other concurrent disease, the diagnosis should be made particularly cautiously, and based on a combination of supportive history, physical examination and clinicopathological findings, diagnostic imaging findings, endocrine test results and response to treatment. Practically, this means attempted management of DM is usually necessary before diagnostic testing of HAC can be attempted.
In patients with concurrent DM and HAC, the treatment of DM should be started promptly to avoid the development of ketoacidosis and/or severe hyperglycaemia. However, DM remains poorly controlled in the majority of these dogs – despite insulin therapy – and good glycaemic control is generally not possible until the HAC is well controlled5.
Although insulin treatment is recommended, aggressive efforts to obtain a tight control of DM are discouraged because the insulin resistance caused by HAC will hamper achieving a good glycaemic control. Moreover, once HAC is treated, increased insulin sensitivity and a decreased need for insulin may lead to hypoglycaemia.
The initial management and stabilisation of a diabetic patient is beyond the scope of this article, but the authors suggest starting with a conservative dose (approximately 0.25 IU/kg to 0.5 IU/kg) of intermediate-acting insulin, which should initially be administered twice a day5. For most dogs with concurrent DM and HAC, glycaemic control remains poor, despite increasing insulin doses.
Given insulin resistance is likely to be encountered, once the diagnosis of both conditions has been made, the main aim should be managing the HAC, while achieving adequate control of the diabetes. Categorisation into pituitary or adrenal-dependent HAC is important to determine treatment options, and should be diagnosed as recommended in non-diabetic patients (for example, endogenous ACTH and imaging studies)6.
Whether the management of HAC should be altered due to the presence of concurrent DM has not been well studied; the authors considered both medical or surgical (hypophysectomy or adrenalectomy) management, as would be recommended in a non-diabetic patient. As the licensed product and most commonly used treatment, the authors prefer the use of trilostane for medical management of pituitary-dependent HAC. In an attempt to maintain consistency with the insulin dosing, and reduce variability, the authors prefer twice-daily dosing in diabetic patients, and would recommend an initial dose of 0.5mg/kg to 1mg/kg every 12 hours.
While monitoring for improvements in clinical signs is important for assessing treatment response, it must be remembered clinical signs common to both diseases, such as polyuria/polydipsia or polyphagia, may not be useful because they will continue until both endocrine diseases are well controlled, and persistence of these signs will not help differentiate the cause. Therefore, despite recognised limitations, the use of repeat ACTH stimulation tests is recommended to determine when the HAC is appropriately treated.
Once the HAC is adequately managed (ideally with a cortisol concentration between 30nmol/L to 150nmol/L pre-ACTH and post-ACTH, three to four hours after trilostane dosing), the insulin resistance is expected to resolve, unless another concurrent disease is present (for example, UTI). Once adequate HAC control has been achieved, adjustments of the insulin dose to achieve a good glycaemic control can be started.
Alternative methods for monitoring trilostane therapy, such as the pre-pill and three-hour post-pill basal cortisol, have shown promise for monitoring the clinical response to therapy, with suggestion this method may be superior to ACTH stimulation testing. The authors believe evidence is insufficient to support the sole use of this method for monitoring dogs with concurrent HAC and DM, although it may prove useful in the future7.
Although adequate treatment of HAC is important to achieve a good glycaemic control, the dose of insulin may not decrease. In a small study, with eight dogs with concurrent DM and HAC treated with trilostane, insulin requirements were not consistently reduced on management of HAC – although three dogs received trilostane once a day8.
It is important for clinicians and owners to be aware canine DM, unlike the feline disease, will never enter remission with treatment of HAC, even if that disease appears to be the cause of the diabetes. Canine DM will require life-long insulin therapy.
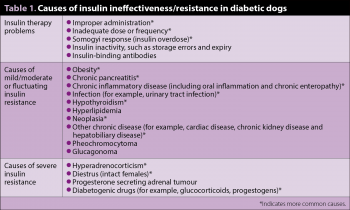
Other endocrine diseases can occur alongside patients with DM, such as part of a polyglandular syndrome or coincidentally/sporadically. Consideration for their presence should be particularly considered in patients with clinical signs that cannot be attributed to DM, or with evidence of mild to severe insulin resistance.
Possible causes of insulin-resistant DM dogs are outlined in Table 1.
The combination of hypoadrenocorticism and hypothyroidism is likely the most common autoimmune polyglandular syndrome recognised in dogs1. This combination of hypoadrenocorticism and hypothyroidism (with or without type-one DM and other endocrine insufficiencies) is a recognised syndrome in humans, known as Schmidt syndrome or autoimmune polyendocrine syndrome type-two.
A case report has documented the combination of autoimmune hypoadrenocorticism and hypothyroidism in a three-year-old Dobermann pinscher, with clinical features and serological testing supportive of this case being similar to the human syndrome9. Although no attempt was made to confirm an autoimmune aetiology, a previous study found 4% of dogs with hypoadrenocorticism had concurrent hypothyroidism10. Hypoadrenocorticism is usually diagnosed first, based on an inadequate adrenal response to an ACTH stimulation test11.
Dogs with hypoadrenocorticism receiving mineralocorticoid and glucocorticoid supplementation should improve rapidly. Therefore, concurrent hypothyroidism should be suspected and thyroid function tests performed in dogs with hypoadrenocorticism displaying a poor response to mineralocorticoids, persistent hyponatraemia or hypercholesterolaemia, dermatological signs, obesity, bradycardia and/or heat-seeking behaviour11.
The measurement of canine thyroid stimulating hormone (cTSH), along with total thyroxine (T4) and free T4, is recommended in these cases to achieve a diagnosis of hypothyroidism, given the effects of the concurrent disease and therapy on assessment of thyroid function. A total T4 within or above the reference range makes a diagnosis of hypothyroidism unlikely, although the presence of thyroid hormone autoantibodies can interfere with this test. However, a low total T4 is non-specific, as concurrent diseases and medications, including glucocorticoids, can decrease total T4 concentration. For the diagnosis of hypothyroidism, free T4 concentration measured by equilibrium dialysis is more specific than total T4, but severe non-thyroidal disease can still decrease free T4 concentration.
The cTSH would be expected to be increased in cases of primary hypothyroidism, and a combination of low total T4 or free T4 and increased cTSH is highly specific for hypothyroidism. However, up to 38% of hypothyroid dogs have cTSH concentrations within the reference range and cTSH concentration may also be increased in dogs with normal thyroid function12. Therefore, in a dog with suspected hypothyroidism and a low total T4 or free T4 – but a cTSH within the normal limits – performing a thyroid stimulating hormone (TSH) stimulation test or thyroid scintigraphy can be considered. Alternatively, a therapeutic trial can be considered, and, it is, many times, the most practical approach to confirm a diagnosis of hypothyroidism. If therapy leads to an improvement of clinical signs within an appropriate time frame, treatment should be temporarily discontinued to determine if clinical signs recur, which would be compatible with hypothyroidism.
Dogs with concurrent hypothyroidism and hypoadrenocorticism should have the latter disease managed first, and the authors’ first choice treatment for clinically stable, chronic cases would be desoxycorticosterone pivalate and prednisolone at standard doses.
Treatment of acute Addisonian crisis should be undertaken, if necessary, and would be the same as in a dog with no suspicion of comorbidities; it is beyond the scope of this article to detail the emergency management of this scenario.
Hypoadrenocorticism is life-threatening, meaning its management should always take priority. In addition, levothyroxine supplementation increases the basal metabolic rate, which may exacerbate electrolyte imbalances in the unstable dog with hypoadrenocorticism. However, hyponatraemia may never completely resolve until the hypothyroidism is treated.
Once the hypoadrenocorticism is stable, treatment with synthetic sodium levothyroxine can be prescribed at an initial standard dose of 0.02mg/kg twice a day, with progressive dose increments according to the clinical signs and therapeutic monitoring. It is not known what the appropriate therapeutic range in hypothyroid dogs with concurrent diseases is, but it is likely to be lower than the range for otherwise healthy dogs11.
Although not routinely recommended in a dog that has clinically stabilised, in some cases, measurement of cTSH along total T4 may help provide treatment recommendations, as a persistently elevated TSH indicates inadequate supplementation or poor compliance.
The development of hypothyroidism in diabetic patients appears to occur more often than development of DM in hypothyroid dogs, with an estimated prevalence of approximately 4% to 9% and 1.2% to 1.5%, respectively2.
Thyroid hormones are important for glucose homeostasis, and hypothyroidism may cause insulin resistance in some diabetic dogs12. Compared to HAC, concurrent hypothyroidism is likely to cause only mild insulin resistance. Furthermore, the fructosamine concentrations in hypothyroid dogs can be increased, even if they are not diabetic, due to decreased metabolic rate and resultant decreased protein turnover, which may complicate the assessment of glycaemic control in hypothyroid and diabetic dogs13.
The diagnosis of hypothyroidism in poorly controlled diabetic dogs is also challenging because many clinical signs (for example, lethargy and weakness) and clinicopathological abnormalities (for example, hypercholesterolaemia) are shared by both diseases12.
Given the effect of non-thyroidal illness on thyroid function tests, it is generally recommended to try to stabilise DM, before thyroid testing is performed, although a good glycaemic control may not be achieved due to the insulin resistance caused by the concurrent hypothyroidism. The interpretation of the thyroid function results is challenging and should take into consideration the degree of success in controlling hyperglycaemia; it would be expected the poorer the glycaemic control achieved, the more severe decreases in thyroid hormones, particularly total T4 (due to the concurrent DM) will be. The diagnosis of hypothyroidism should never be made on the basis of total T4 alone. In dogs with concurrent DM, the measurement of cTSH and free T4, along total T4, is recommended as cTSH and free T4 are less severely affected by a concurrent disease than total T4.
Diabetic dogs newly diagnosed with hypothyroidism should have their blood glucose concentration closely monitored after starting levothyroxine supplementation until both diseases are well controlled because once euthyroidism is restored the insulin sensitivity will increase, which may lead to hypoglycaemia. Clinicians should be mindful hypoglycaemia is likely to be more acutely life-threatening than hyperglycaemia and underdosing of insulin is likely to be safer than risking overdose during the stabilisation of these patients.
As medical veterinary management improves, it is likely concurrent endocrinopathies will be recognised with increasing frequency. While they are not common, the presence of concurrent endocrine diseases can significantly complicate the management of both conditions. This is particularly evident in the stabilisation of DM, where insulin sensitivity can be altered, making management frustrating, unless the concurrent disease is recognised and treated. However, clinicians must also be mindful of the difficulty in interpreting diagnostic testing in the scenario of multiple endocrinopathies and resist the temptation to overdiagnose these uncommon comorbidities.
When approached carefully, the recognition and treatment of dogs with multiple endocrine diseases will lead to improved clinical care, better quality of life for the patient and much greater satisfaction for both the clinician and pet owner.