8 May 2017
James Warland and Jason Bestwick discuss disease entities and treatments, including diet and nutraceuticals, with an emphasis on the lower urinary tract.

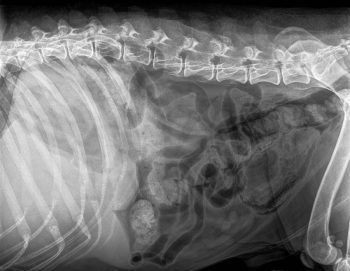
Figure 2. Right lateral plain abdominal radiograph of a dog with emphysematous cystitis. Note the presence of gas in the bladder wall.
Cystitis – inflammation of the wall of the urinary bladder – is a common problem seen in canine patients in general practice, with 14% of all dogs acquiring a bacterial cystitis in their lifetime1.
Unlike cats, in which most cases of cystitis are idiopathic and sterile, bacterial infection is the most common cause in dogs. Typical clinical signs include dysuria/stranguria, haematuria and pollakiuria. Pollakiuria was the most common clinical sign reported by Westropp et al2. However, some bacterial urinary tract infections (UTIs) can be asymptomatic.
Other causes of cystitis include urolithiasis/crystalluria, bladder neoplasia and prostatic disease, and can be drug-induced. Some cases can develop profound wall changes, such as is seen in polypoid and emphysematous cystitis.
Initial investigations will depend on the signalment and clinical exam findings. In most cases, the initial investigations are urinalysis and urine culture.
Bacterial culture and antibiotic sensitivity testing are considered the gold standard in diagnosing a UTI and, of course, help direct appropriate antibiotic treatment. The most commonly isolated bacteria in cases of canine UTIs are Escherichia coli, followed by Streptococcus species3,4. If outward signs of comorbidity exist, such as an endocrinopathy – for example, hyperadrenocorticism or diabetes mellitus – then specific testing for these conditions is warranted. Imaging of the urinary tract is also recommended in atypical or recurrent cases.
It is important to recognise urine culture will not aid in discerning between a UTI and an asymptomatic bacteriuria. In one study, the prevalence of asymptomatic bacteriuria was 25% in morbidly obese, but otherwise healthy, dogs5.
The treatment of asymptomatic UTIs in dogs is still a contentious subject in veterinary medicine, with limited data to inform on appropriate management. In human medicine, most asymptomatic infections are not treated, but this can depend on individual patient factors – for example, pregnant women are often treated due to the risk of development of pyelonephritis and subsequent impacts on their unborn child6.
The distinction between asymptomatic bacteriuria and a UTI can be made on the basis of present clinical signs and may be assisted by a urine sediment examination, which should be performed as soon as possible after sample collection, as cells and casts will rapidly start to degrade and bacteria may multiply.
It should be noted different patients will display clinical signs to different extents, while owners may be variably reliable in their observation of their pet’s clinical signs.
Most often, diagnostic samples for culture must be posted to the laboratory and, thus, emerges the question of which tube to use – plain, or with boric acid. Evidence suggests using a plain, sterile tube is preferable, with greater sensitivity and similar specificity to cultures obtained from samples in boric acid tubes for samples collected in a sterile manner, via cystocentesis7.
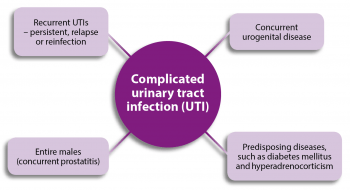
An uncomplicated, or simple, UTI has no underlying cause – the dog is otherwise healthy, with a normal urogenital tract – and does not occur more frequently than every four to six months.
In complicated UTIs (Figure 1), an underlying abnormality exists that predisposes the dog to develop a UTI or fail treatment. This can include abnormal function or anatomy of the urogenital tract, or concurrent diseases, all of which may predispose the dog to acquire a UTI or make resolution difficult.
Due to the difficulties in treating UTIs – in particular, complicated UTIs – they may not resolve (persistent infection) or may recur (relapse or reinfection).
Persistent infection, in the face of appropriate antibiotic selection based on culture and sensitivity, infers the development of antibiotic resistance or inadequate concentrations of the antibiotic being achieved in the urine. Owners should be consulted to ensure compliance is not the cause of persistent infection. Thought should also be given to the pharmacokinetics and availability of the selected antibiotic in the urine, alongside any diseases that may reduce its availability, such as gastrointestinal (decreased absorption) and renal disease (decreased urinary excretion), or prostatic disease (lack of tissue penetration).
In a relapse, a negative urine culture and resolution of clinical signs are documented after appropriate antibiotic treatment, but infection recurs with the same causative agent shortly – usually days to weeks – after cessation of treatment. This relapse happens due to residual bacteria in the urogenital tract, not cleared by the antibiotic course, recolonising in the urinary system. This may occur due to insufficient length of antibiotic course or underlying disease creating a nidus of infection.
In cases of reinfection, the causative bacteria is eradicated with appropriate treatment, but, due to an underlying susceptibility of the patient to acquire a UTI, subsequent infection is caused by a different species or strain of bacteria. For dogs that encounter frequent reinfection, full clinical investigations are indicated to ascertain the cause of increased predilection for developing a UTI. This could include routine haematology and biochemistry, specific disease testing (for example, for hyperadrenocorticism), imaging (for example, ultrasound, plain radiography and contrast studies) and cystoscopy.
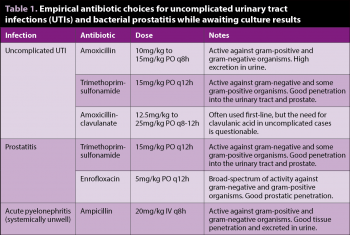
Dogs that present with acute clinical signs and initial in-house testing (dipstick/sediment examination) consistent with a simple UTI often require empirical antibiotic treatment while awaiting culture results (Table 1). Given concerns about the responsible use of antimicrobials, the authors recommend urine culture and antimicrobial sensitivity testing are performed in all patients. Culture and sensitivity testing become mandatory in complicated cases and cases of treatment failure. Antibiotic choice should be made on factors including spectrum of activity and effect against the suspected bacterial agent, penetration into target tissues, and patient-specific contraindications.
The authors’ recommended first-line antibiotics are amoxicillin or trimethoprim-sulfonamide10. If the chosen antibiotic is proved efficacious by the results of culture and sensitivity testing then the course is typically continued for seven days10. In simple UTIs, repeated urinalysis and urine culture do not need to be performed if clinical signs resolve with appropriate treatment.
It is important to note, if clinical signs are improving, it may be prudent to continue the course, even if the bacteria cultured is shown to be resistant to the empirical antibiotic chosen. This is because antibiotic concentrations in the urine often reach substantially higher concentrations than those tested in the laboratory. In this circumstance, it is useful to repeat urinalysis and culture after cessation of antibiotic therapy to ensure resolution.
Historic recommendations for canine cystitis have included longer courses (7 to 14 days) of antibiotics for uncomplicated cystitis10. Much shorter courses (three days) of antibiotics, such as trimethoprim or fluoroquinolones, are often used in human patients with uncomplicated UTIs11. The use of high-dose, short-duration treatment with enrofloxacin (18mg/kg to 20mg/kg PO SID for 3 days) has been assessed in the treatment of uncomplicated UTIs in dogs and was shown to not be inferior to more conventional treatment with amoxicillin-clavulanate (13.75mg/kg to 25mg/kg PO BID for 14 days)2. Clinical and microbiological cure rate, with repeated urine culture, were assessed and not significantly different between both groups. Further studies into short-duration antibiotic treatment in veterinary species are required before recommendations can be made.
In the authors’ practice’s antibiotic guidelines, the use of higher-tier antibiotics – including fluoroquinolones – is reserved for cases with documented antimicrobial sensitivity and an expectation a first-line antibiotic will not suffice, either due to bacterial resistance or poor tissue penetration. They recommend all practices subscribe to good antibiotic stewardship protocols. Support for practices to produce guidelines are available, such as those produced by the BSAVA Protect scheme12.
Initial diagnosis and empirical treatment, if required, is similar to that with uncomplicated UTIs. Urine bacterial culture and antimicrobial sensitivity testing must be performed in complicated cases. In entire male dogs, prostatic penetration should be considered. Efforts should be made to identify and, where possible, address any underlying causes – for example, uncontrolled diabetes mellitus or urolithiasis.
No evidence to suggest appropriate treatment length is available; four to six weeks is typically recommended, but may be altered by specific patient factors and the underlying cause identified. In complicated cases, urine culture should be performed 7 days after starting treatment and 7 to 14 days after stopping to assess the success of therapy10.
In recurrent or persistent infections, clinicians should focus on trying to find an underlying cause. If an underlying cause is identified, it is likely to be impossible to adequately resolve the problem without identifying and treating this cause. Suggested considerations are given in Panel 1.
Emphysematous cystitis (Figure 2) is caused by infections with gas-forming bacteria – most commonly E coli – and described in sporadic case reports in veterinary literature. Cases have mostly been reported in diabetic and glucosuric patients, but have been described in patients with chronic UTIs and in one patient receiving long-term prednisolone therapy secondary to infection with Clostridium perfringens. Prognosis appears to be good with appropriate antibiotic treatment. Intravesiculal instillation of formalin has also been described as a treatment13.
Polypoid cystitis (Figures 3 and 4) is typified by the formation of intraluminal benign epithelial growths, usually located on the cranioventral aspect of the bladder wall. The distribution is typically different to that seen with neoplasia, but confirmation of the diagnosis should be made histologically – for example, via catheter suction biopsy or cystoscopic biopsy. Fine-needle aspirates or cystocentesis should not be performed until neoplasia has been ruled out.
Proteus species are the most common bacteria isolated in these cases14, but others have been described. Antimicrobial therapy can be tried, but the polyploid changes may mean clearance of bacterial infection is challenging.
In the authors’ experiences, clearance of the UTI may be achieved with prolonged (six weeks) antimicrobial therapy, and despite the polyploid changes not fully resolving, clinical signs may not recur and bacterial clearance may be demonstrated. In cases where bacterial clearance is not possible due to the anatomical changes, partial cystectomy seems to be the treatment of choice; however, information is limited14.
Transitional cell carcinoma (TCC) is the most common neoplasia of the canine bladder15. Clinical signs can be similar to those reported in cystitis, including dysuria, haematuria and pollakiuria. Diagnosis may be suspected based on clinical signs, signalment and characteristic location of the bladder mass as seen on ultrasonography; TCC has a predilection for the trigone.
Definitive diagnosis is made by histopathology obtained by cystoscopy or ultrasound-guided suction biopsy. The authors advise against fine-needle aspiration of bladder masses, due to the risk of seeding tumour to the abdominal wall.
Prognosis is poor, as the tumour is difficult to resect surgically, while response to chemotherapy is variable. The use of cyclo-oxygenase 2 inhibiting drugs and radiotherapy are being further investigated15.
Urethral stents may be used in cases causing urinary obstruction16; consultation with a veterinary oncologist is recommended.
Cyclophosphamide, a chemotherapy agent used to treat canine lymphoma as part of the cyclophosphamide, vincristine and prednisolone protocol, and the cyclophosphamide, doxorubicin, vincristine and prednisolone protocol, may cause sterile haemorrhagic cystitis.
Strategies to try to reduce the incidence of haemorrhagic cystitis include concurrent treatment with furosemide and dividing the desired dose over a three-day period17.
Regular monitoring of cases receiving cyclophosphamide with urinalysis, to check for microscopic haematuria, is recommended to prevent this complication becoming severe. Although bacterial infection is a possible complication, these cases are typically sterile, so antibiotic therapy is not indicated. Consultation with a specialist oncologist is recommended.
A disease, typically, of older entire male dogs, prostatitis is usually caused by an ascending bacterial infection and, most often, develops secondarily to another prostatic disease process such as neoplasia or benign prostatic hyperplasia18.
Dogs with acute prostatitis usually present with systemic signs of illness, such as pyrexia, lethargy and anorexia, but chronic cases are also seen and may show few clinical signs. A rectal examination should be performed to assess the prostate for size, shape and pain.
Imaging with radiography and abdominal ultrasound, alongside prostatic wash, are the mainstay diagnostic procedures. Urinalysis and urine culture are recommended in these cases, as a concurrent bacterial cystitis is possible and results between urine and prostatic culture appear to correspond well (Table 1)19.
Antimicrobial therapy of prostatitis is challenging due to poor tissue penetration of some antibiotics, including penicillins, and must be considered when antibiotic selection is made. Trimethoprim-sulfonamide, fluoroquinolones, clindamycin and doxycycline all penetrate the prostate well, but choice should be based on culture results. Management of the underlying prostatic disease with either medical – for example, osaterone – or surgical treatment is also mandatory to control prostatic disease.
The cause for formation of stones within the urinary tract is multifactorial, including patient factors – genetic, metabolic and anatomical – and diet. Although some crystals/uroliths may be caused by UTIs, in the case of struvite crystals, the converse may also be true – uroliths may predispose the patient to a UTI or form a nidus of infection preventing successful clearance of the organism.
Lower urinary tract signs are common in dogs with urolithiasis, due to a resultant cystitis. A basic work-up includes urinalysis and imaging of the urinary tract with ultrasonography and radiography, with or without contrast studies. Many options are available for the treatment of urocystoliths, depending on the type and size, including surgical removal, medical dissolution, voiding urohydropropulsion and lithotripsy20. A full discussion of urolithiasis is beyond the scope of this article.
Inflammation of the kidney and renal pelvis is usually caused by an ascending bacterial infection in dogs. It may be seen concurrently with cystitis. Typical lower urinary signs, such as dysuria, may be absent or precede upper urinary tract signs, such as polyuria and polydipsia, and abdominal pain alongside possible systemic signs, such as pyrexia and lethargy. Urinalysis (urinary casts) and urine culture are crucial in diagnosing pyelonephritis; this may be performed on samples acquired via cystocentesis or pyelocentesis. Ultrasound findings may also support the involvement of the kidneys.
Acute pyelonephritis patients usually require hospitalisation for initial management, often with IV fluid therapy (Table 1) and parenteral antibiotics. Antimicrobial therapy usually lasts a minimum of four to six weeks21, and clearance of infection should be confirmed with repeated urine cultures before and after halting treatment.
Although sometimes employed in human medicine, the use of prophylactic antibiotic therapy is hotly debated. Evidence for its use in veterinary medicine is absent and raises concern over potential side effects and antimicrobial resistance. Therefore, its use is not recommended, other than in exceptional circumstances; the authors recommend discussion with an internal medicine specialist before considering this approach.
Methenamine hippurate has been used prophylactically in human medicine for recurrent cystitis22. It works through its conversion to formaldehyde within acidic urine (urine-acidifying agents are administered alongside methenamine in human patients), which may be bacteriostatic or bactericidal depending on the concentrations reached within the bladder. In human patients, it seems it may be effective, at least in the short term, when used for UTI prophylaxis; however, a paucity of data exists on its use23. It is contraindicated in renal disease and no evidence or recommendations for their use exist in veterinary medicine.
Proanthocyanidins, the active substance in cranberry extract, which prevents the adhesion of E coli to the urinary epithelial cells, have been scarcely studied in canine patients. One clinical trial found no significant reduction in the prevalence of bacteriuria, by administering oral cranberry extract, in dogs undergoing surgery for acute thoracolumbar intervertebral disc herniation24. However, a small study has demonstrated promising results in vivo, preventing development of UTIs in patients with a history of recurrent UTIs; and in vitro, with reduced E coli adherence to canine kidney cells when incubated with urine from treated dogs25. In human medicine, studies have failed to demonstrate convincing evidence for their use and a 2012 Cochrane systematic review concluded they could not be recommended for the prevention of UTIs26.
Instillation of tromethamine-ethylenediamine-tetraacetate solutions, used in conjunction with parenteral or IV antibiotics, into the urinary bladders of dogs with multidrug-resistant or recurrent UTIs has been described in a case series27 and case report28. Promising results were demonstrated, with improvement of clinical signs and negative bacterial cultures obtained post-treatment. No side effects were reported. This promising approach should be considered in challenging cases, but larger studies are needed to demonstrate efficacy.
Other treatments and preventive strategies occasionally discussed include the use of glycosaminoglycan supplements, instilment of non-pathogenic E coli into the bladder to compete with urinary tract pathogens (persistent bacteriuria has not been successfully achieved29), probiotics, and the use of oestrogen to alter the vaginal environment in females. A prospective controlled study evaluated the efficacy of an oral probiotic supplement, for a period of either 14 or 28 days, to alter vaginal flora in normal spayed female dogs. Oral supplementation failed to increase the prevalence of vaginal Lactobacillus, which was hypothesised to be beneficial30.
These treatments and attempts at prophylaxis lack evidence in the veterinary medicine literature, but present research opportunities. With the evidence available, the authors are not able to make clear recommendations regarding the use of nutraceuticals, but they may be considered in recurrent or challenging cases. As aforementioned, the authors would recommend a full investigation of potential underlying causes alongside instigating supplementary therapies.
Canine cystitis may have many underlying causes. Regarding bacterial infections, the majority of UTIs encountered in general practice are likely to represent uncomplicated UTIs; however, the presence of possible complicating factors should be considered on an individual patient basis. An unusual signalment, recurrence or persistence of an infection should prompt careful history taking, including questioning compliance, and the search for underlying diseases and predisposing factors. The importance of urinalysis and urine culture in all cases cannot be overemphasised.
The authors are grateful to Mayank Seth, BVetMed, BSc, DipACVIM, DipECVIM-CA, MRCVS for reviewing the manuscript and his advice.