23 Sept 2021
Anais Allen-Deal BVetMed, MRCVS and Daria Starybrat MRCVS describe the diagnosis of this issue in cats and dogs, as well as medical and surgical management, and prognosis.

Pyothorax – or empyema as it is sometimes known – is a pleural space disease affecting both cats and dogs (Demetriou et al, 2002).
Underlying causes – such as migrating foreign bodies, penetrating thoracic trauma, pneumonia and haematogenous spread – can lead to the buildup of purulent material within the thoracic cavity (Silverstein and Hopper, 2014; Demetriou et al, 2002).
Common presenting signs for cats and dogs with pyothorax include dyspnoea and tachypnoea, pyrexia, lethargy, hyporexia/anorexia and weight loss (Demetriou et al, 2002).
On clinical exam, dyspnoea with muffled heart and respiratory sounds should prompt clinicians to perform thoracic imaging.
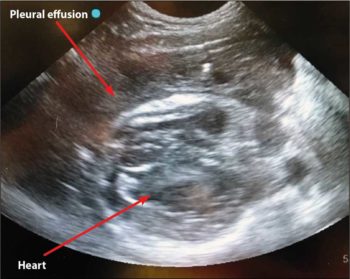
Thoracic point-of-care ultrasound can be used to rapidly identify pleural effusion in cats and dogs. This can also be performed kennel‑side, with minimum restraint and stress to the patient (Hamel and Berry, 2018).
Purulent fluid can often be very echogenic on ultrasound and may, in some cases, be confused with a diseased lung (Figure 1).
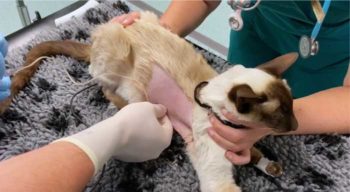
Thoracocentesis is the next step to both diagnose and stabilise the patient (Figure 2). Initial cytology of the fluid can be performed in‑house and is typically consistent with a septic inflammatory exudate (Figure 3).
In some patients, especially those that are currently being treated with antibiotics, cytology may show a heavily inflammatory exudate without the presence of intracellular bacteria (Demetriou et al, 2002). This should still be considered as highly suspicious of pyothorax.
In all patients, a fluid sample should be submitted to an external laboratory for evaluation of cytology, and culture and sensitivity (Barrs and Beatty, 2009a; 2009b). However, treatment with broad‑spectrum antibiotics and management of the patient should be initiated while results are pending to improve outcomes.
Once the patient is stabilised and pleural effusion drained, three-view thoracic radiographs can be performed to identify obvious mass lesions or pulmonary disease.
While both radiographs and ultrasound are effective at identifying pleural effusion, lesions such as abscesses can be missed. Research has suggested a good agreement between CT and surgical findings in dogs (Swinbourne et al, 2011). This has not been fully investigated in cats, so the utility of CT in feline pyothorax is unknown.
Medical management is often an appropriate first approach. Thoracic drainage – either by repeat thoracocentesis or the placement of thoracostomy tubes – allows for the relief of dyspnoea while also reducing the nidus of infection.
An improved prognosis is reported when thoracostomy tubes are used as opposed to repeat thoracocentesis (Barrs and Beatty, 2009a; 2009b). Thoracostomy tube placement via a modified Seldinger technique is a relatively simple procedure that can be performed under sedation or general anaesthesia (Panel 1).
Thoracostomy tubes can also allow for lavage to be performed. Lavage of the chest has been found to reduce the length of thoracostomy tube requirement, although further research is required to clarify the benefit of lavage in these patients and the best protocol to perform it (Demetriou et al, 2002).
Current recommendations are to lavage the thorax every 4 to 12 hours with warmed physiological saline for the first 24 to 48 hours (Barrs and Beatty, 2009a; 2009b; Silverstein and Hopper, 2014).
During lavage, gloves and chlorhexidine or alcohol wipes should be used to wipe the needle free valve to maintain asepsis and prevent introduction of further bacteria to the pleural space. Care should also be taken to prevent introduction of air to the pleural space.
Lavage can be continued if the fluid continues to be flocculent after 48 hours.
Thoracic ultrasound prior to removal is recommended to ensure no remaining pockets of fluid are present.
Appropriate antibiosis is essential for good patient outcome. Generally, starting with broad‑spectrum antibiotics – for example, amoxicillin-clavulanic acid – via an IV route is preferred.
In patients that have not responded to initial broad-spectrum antibiotics, fluoroquinolones – such as enrofloxacin or marbofloxacin – can be considered. Enrofloxacin should be avoided in cats as it can cause retinal degeneration (Gelatt et al, 2001).
However, a culture and sensitivity profile must be submitted prior to escalation, and where possible, antibiotic choice should be guided by these results. Common bacteria identified include Escherichia coli, Pasteurella species, Nocardia species and Actinomyces species (Demetriou et al, 2002).
A four to six-week course of antibiotics has been previously recommended.
Imaging, such as thoracic radiography, should be repeated four weeks after discharge to assess for recurrence and prior to stopping antibiotics.
In the variety of cases, pyothorax can lead to pleuritis and, therefore, be a painful condition. Additionally, the placement and maintenance of thoracostomy tubes can lead to discomfort for the patient.
Analgesia is, therefore, essential for the animal’s welfare.
A variety of analgesic drugs are appropriate for use in pyothorax, including opioids such as methadone or buprenorphine, and paracetamol (dogs only). Analgesic options are well covered in Walsh (2019).
It would be pertinent to avoid the use of NSAIDs as some patients with pyothorax can descend into sepsis and NSAIDs can contribute to, or cause, an acute kidney injury (Walsh, 2019).
Fluid therapy can be challenging in any patient with thoracic disease. On presentation, most dogs and cats with pyothorax will be dehydrated, and in some cases, hypovolaemic.
When hypovolaemia is detected, appropriate boluses of fluid therapy (5ml/kg to 10ml/kg bolus of isotonic crystalloids, such as lactated Ringer’s solution, given over 10 to 15 minutes) can be used to improve the perfusion parameters (heart rate, mucous membrane colour, capillary refill time, pulse strength and quality, and mentation).
Some patients may experience septic shock and require vasopressors to maintain appropriate arterial blood pressure.
Once hypovolaemia is corrected, an estimate of dehydration can be made and added to maintenance, then corrected over 6 to 24 hours. Most patients would benefit from having their hydration corrected more slowly, then rapidly.
Ongoing losses can be quite considerable in patients with pyothorax due to the exudative nature of their disease. Additionally, fluid instilled during lavage can be absorbed via the pulmonary tissue and overlooked as a source of fluid (Silverstein and Hopper, 2014).
Once hydration is achieved, an assessment of “ins” and “outs” should be made every 12 to 24 hours to maintain adequate hydration without fluid overload (Table 1).
Hydration can be monitored with regular assessment of clinical examination, bodyweight and urine specific gravity.
As with any sick patient, nutrition is essential for good and quick recovery, and should be considered in patients as soon as they are stable.
Ideally, voluntary intake should be encouraged by minimising stress in the environment, using antiemetics (for example, maropitant or ondansetron) to prevent nausea as well as appropriate analgesia.
Some patients may need further support – for example, a nasoesophageal/nasogastric tube or oesophagostomy tube – to provide nutrition. Readers are referred to a previous article where provision of nutrition for critically ill patients was discussed (Weeth, 2016).
Further supportive care can be guided by routine diagnostics, such as haematology, biochemistry and blood gas analysis.
Common secondary conditions can develop, such as hypoglycaemia, anaemia and hypoalbuminaemia.
Hypoglycaemic patients should have this corrected with appropriate glucose boluses – in some cases, infusions of 2.5% or 5% glucose infusions are required.
Anaemic patients should be monitored closely, as, uncommonly, blood transfusion may be warranted.
Hypoalbuminaemia can occur secondary to protein loss and acute inflammation. These patients benefit from judicious fluid therapy and early nutrition to prevent further deterioration.
The majority of dogs are candidates for surgical management. This is thought to be due to the increased likelihood of migrating foreign bodies, although foreign material is not always identified at surgery (Demetriou et al, 2002).
Criteria for surgical management are detection of a pulmonary or mediastinal abscess, extensively loculated effusions and failure of medical management (Barrs and Beatty, 2009a; 2009b).
A referral for median sternotomy is generally recommended to allow for full exploration of the chest, although these have been associated with a high rate of wound infections (Tattersall and Welsh, 2006).
In some patients, findings may be unilateral and a lateral thoracotomy is appropriate in these patients.
Following surgery, a similar protocol as for medical management can be followed for the postoperative period.
One paper reported a survival rate of 95% in cats managed medically with thoracostomy tubes; however, this does not include cats that died prior to thoracostomy tube placement (Barrs et al, 2005).
In dogs, surgical management is reported to have better outcomes (Silverstein and Hopper, 2014).
Both dogs and cats can suffer recurrence, and follow‑up imaging is recommended prior to stopping antibiotics.
Pyothorax in cats and dogs can be both a challenging and rewarding condition to treat.
Patients require 24-hour care for a successful outcome, and some cases may benefit from advanced imaging and surgery.
Referral for dogs and cats that fail initial medical management may be beneficial.
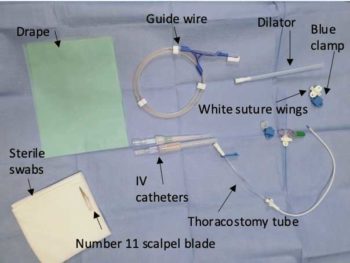
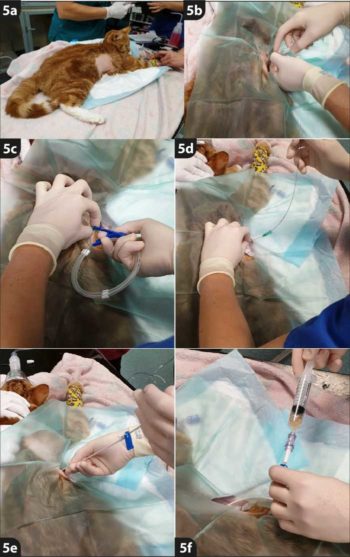
PANEL 1. Placing a thoracostomy tube using modified Seldinger technique (Figure 4)
Equipment checklist (Figure 5):
Process: