23 Jan 2017
Carolina Arenas explains the investigative, treatment and follow-up protocols carried out in a miniature schnauzer emergency case

A 5.7-year-old, female, entire miniature schnauzer was referred to The Queen’s Veterinary School Hospital (QVSH) as an emergency due to sudden onset of vomiting and lethargy that began three days before referral.
Investigations at the referring vet practice revealed marked azotaemia (urea 46.4mmol/L, range 2.5 to 9.6; creatinine 596umol/L, range 44 to 159) and other biochemistry abnormalities (hyperphosphataemia 5.20mmol/L, range 0.81 to 2.20; and hyperproteinaemia 93g/L, range 52 to 82, probably indicating reduced glomerular filtration rate and dehydration; hyponatraemia 123mmol/L, range 144 to 160; and hypochloraemia 87, range 109 to 122, probably secondary to the vomiting episodes with loss of gastrointestinal contents rich in sodium).
Haematology abnormalities included neutrophilia (19.15×10^9/L, range 2 to 12) due to inflammation, stress or infection with increased haematocrit 56.5% most likely due to dehydration.
On the face of these abnormalities, the dog was referred to the QVSH as an emergency. The owners reported they noticed she was nocturic over a 10-day period approximately two to three weeks before referral. She had also been slightly polyuric and polydipsic as they found her drinking from the pool, which she had not done before. They believed the dog had been otherwise healthy until this episode.
On clinical examination, the dog was collapsed. She was very dehydrated (10%) and tachycardic (140bpm), hypothermic (37.6°C) and her body condition score was 5/9 (7.25kg).
Neutrophilia (21.48, range 3×10^9/L to 11.5×10^9/L), hyponatraemia (126, range 135mmol/L to 155mmol/L) and hypochloraemia (80, range 103mmol/L to 120mmol/L) were present, with marked azotaemia (urea 75.5, reference range 2.5 to 7.4; creatinine 776, range 34 to 136) and hyperphosphataemia (7.69, range 0.8mmol/L to 1.73mmol/L).
The dog was normokalaemic (5.4mmol/L, range 3.6 to 5.7) and hyperproteinaemia (93, range 55g/L to 77g/L), increased liver enzymes and high C-reactive protein (54.4, range 0mg/L to 8.2mg/L) were also present. Her anion gap was 41 (range 12mmol/L to 25mmol/L) and bicarbonate was 10.9 (range 18mmol/L to 24mmol/L). All these findings were indicative of acute kidney failure with metabolic acidosis and a systemic inflammatory response.
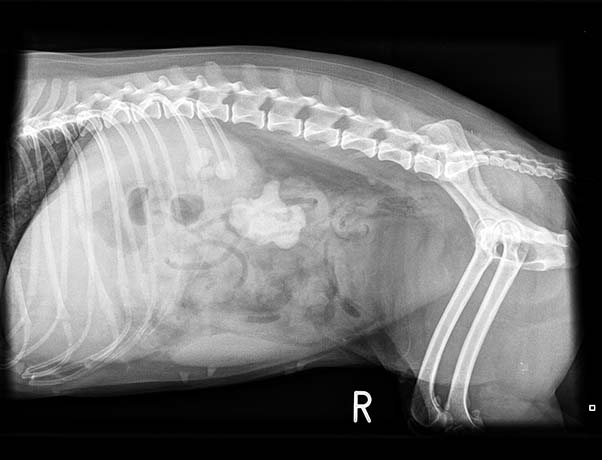
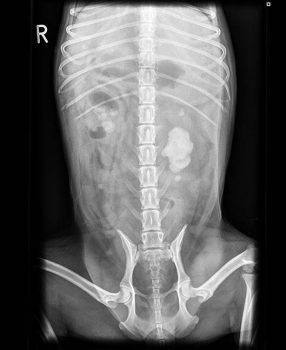
Abdominal radiographs showed bilateral renomegaly (greater than three vertebral bodies). Loss of serosal detail occurred, indicative of peritonitis. The left kidney had mineralised material filling the renal pelvis completely (4.4cm length × 3.1cm height) and a further focus in the proximal ureter. The right kidney had several mineralised structures in the renal pelvis. A small opacity was in the retroperitoneum at the level of L4 to L5 (Figures 1 and 2).
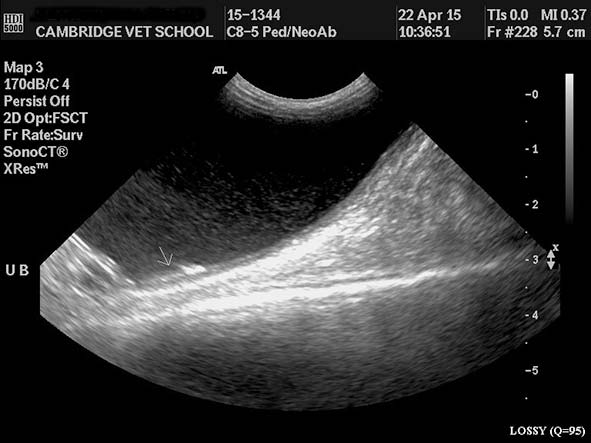
The left kidney was 6.7cm and the right 5.5cm. Both were surrounded by a rim of subcortical hypoechoic fluid and hyperechoic fat (Figures 3 to 5). The right ureter was dilated (Figure 6) and just cranial to its insertion in the urinary bladder was a calculus (Figures 7 and 8). The bladder was filled with highly echogenic material. The uterus and adrenal glands were normal. Free abdominal fluid was present and the peritoneum appeared markedly hyperechoic.
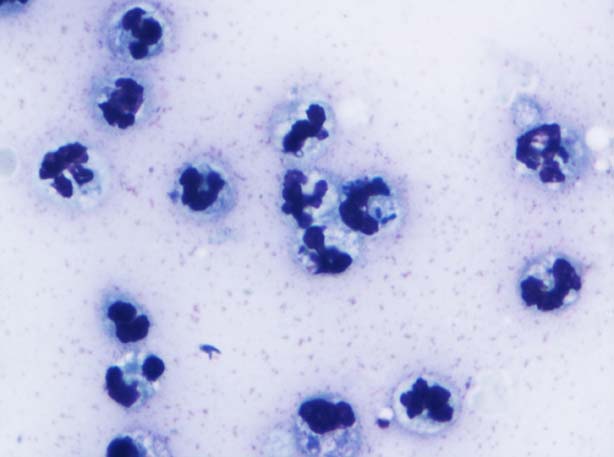
These findings were indicative of acute renal failure (ARF) and bilateral renal calculi formation, and right ureteric partial obstruction and peritonitis. A urine sample taken by cystocentesis revealed presence of gross pyuria – 50 white blood cell count (WBC) per high-power field (HPF), reference range 0 to 5 (Figure 9) – with bacteriuria and haematuria – 10 red blood cell count (RBC) per HPF, reference range 0 to 5.
The urine specific gravity (USG) was 1.016, with a urine protein to creatinine (UPC) ratio of 25.84 (range 0 to 0.4). The isosthenuric urine, despite the presence of ARF, could be either due to underlying chronic failure or pyelonephritis.
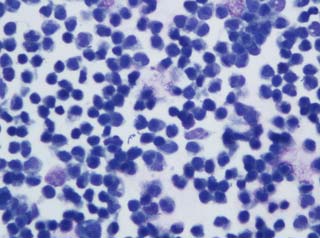
Analysis was consistent with a septic peritonitis (Figure 10); however, the culture did not grow any organisms. The creatinine of the fluid was 830umol/L. This was higher than serum creatinine, although not double, making uroperitoneum less likely.
Gram-positive cocci and Gram-negative rods were seen with the Gram stain. The culture grew Staphylococcus species and Streptococcus sensitive to potentiated amoxicillin, cephalexin, co-trimoxazole and enrofloxacin, and resistant to metronidazole and ampicillin. Although the Gram stain showed Gram-negative rods, no Gram-negative bacteria were cultured.
Basal cortisol was 297nmol/L (range 28 to 250), ruling out hypoadrenocorticism.
Leptospirosis PCR (urine) was negative.
It was initially debated whether to surgically remove the urinary stones (mainly the ureteric stone sitting in the right ureter, which was most likely blocking urinary flow and potentially performing nephrectomy of the left kidney as this kidney was completely filled by a calculi); however, it was decided to stabilise the dog and start treatment for ARF and septic peritonitis. She was started on Hartmann‘s solution, antibiotics (potentiated amoxicillin and marbofloxacin), antiemetics (maropitant), antacids (ranitidine) and analgesia (methadone).
A urinary catheter was placed to monitor urine flow (which was above 2.3ml/kg/hour throughout the first day) and a peritoneal drain was also placed for dialysis and peritoneal lavage for the septic peritonitis. The dog was given a convalescence milk by syringe. On the same day as placement of the peritoneal drainage, the creatinine dropped from 675umol/L to 567umol/L. Three days later, the azotaemia had much improved (creatinine 232umol/L, urea 22.7mmol/L), so it was decided to remove the peritoneal drain.
The dog was continued on IV fluids and supportive medication until the azotaemia had resolved (which was approximately six days after presentation), at which point the fluids were stopped. She started to eat a bland diet (chicken and rice) three days after admission. Her appetite much improved on the fourth day of hospitalisation (eating 8 to 9 out of 10) and a dissolution diet was introduced on the fifth day of hospitalisation. Haematology and biochemistry screens were repeated two days after stopping fluid therapy.
Haematology only showed mild neutrophilia (12.17×10^9/L) and the biochemistry revealed a normal creatinine (133mmol/L) with still high urea (11mmol/L) possibly indicating still reduction in glomerular filtration rate. During hospitalisation, repeat abdominal ultrasound examinations were performed. These showed the calculus initially located in the ureter had moved further and was now located in the urinary bladder (Figure 11) with partial resolution of the dilation of the ureter. The dog‘s weight at discharge was 6.25kg.
The dog was diagnosed with ARF, pyelonephritis and septic peritonitis. It was suspected the most likely aetiology of the ARF was a severe pyelonephritis. However, it is also possible the dog’s left kidney was not functioning properly, as it had a stone that was occupying the whole renal pelvis and part of the proximal ureter, and the calculi located in the right ureter produced a ureteric obstruction leading to ARF (post-renal). The septic peritonitis was believed to be produced either from a small leakage of urine from the ureter or due to translocation of bacteria from the urinary bladder.
Unfortunately, urinalysis did not reveal presence of urine crystals, so the nature of these was unknown at the time of presentation. However, as they were radiodense, they were most likely struvite or oxalate. Most ureteric calculi in dogs are oxalate and miniature schnauzers have an increased prevalence of oxalate calculi, so they were most likely these. However, stones are often mixed and the presence of urinary tract infection (UTI) may have laid down layers of struvite on top of oxalate.
Starting a diet indicated for both oxalate and struvite calculi was ideal in this case. It was decided to reassess the renal calculi once the dog was fully recovered from the ARF and pyelonephritis to determine whether surgical management would be the most appropriate approach. The dog was discharged on marbofloxacin (15mg by mouth every 24 hours), amoxicillin clavulanic acid (125mg by mouth every 12 hours), ranitidine (15mg by mouth every 8 hours) and a wet dissolution diet 9 days after initial presentation.
The dog was rechecked two weeks and approximately one-and-a-half months after discharge. She was clinically improving. Her owners reported she had been doing very well. She was bright and happy, her appetite was good and she was exclusively eating the wet dissolution diet. She had gained some weight (8kg at the latest recheck; body condition score 6 out of 9). She was urinating normally and had no vomiting episodes.
A biochemistry screen was performed (which was unremarkable) and abdominal radiographs (Figures 12 and 13), which showed a decrease in size of the left kidney (6.5cm, previously 7.7cm). The mineralised material in the renal pelvis had markedly reduced in size (3.3cm × 2.2cm) and opacity. Small and ill-defined mineralisations were still present between the last ribs in the lateral view and most likely located in the renal pelvis. Urinalysis (by cystocentesis) was performed and revealed a USG of 1.010.
The UPC ratio was 0.85 (reference range 0 to 0.4; previously 25) with an alkaline pH (8). Despite the diet being acidifying, the pH was still alkaline, which is most likely related to a persistent UTI, as it was confirmed by the presence of pyuria, haematuria and bacteriuria (Gram-negative rods). The urine culture grew multiresistant Gram-negative rods (lactose-fermenting coliform), which were resistant to ampicillin, potentiated amoxicillin, cephalexin, cefuroxime, cefovecin, ceftazidine, co-trinoxazole, enrofloxacin, marbofloxacin, ciprofloxacin, cloxacilin, erythromycin, clindamycin, doxycycline, ticarcillin, oxytetracycline, carbenicilin and metronidazole.
We therefore decided to increase the dose of marbofloxacin (40mg by mouth every 24 hours) to try to increase its urine concentration and the concentration-dependent bactericidal activity, and the dog was maintained on amoxicillin clavulanic acid (same dose).
One month after the last appointment, we rechecked the dog again. She was still doing clinically well and her weight had remained stable. Abdominal radiographs showed the left renal urolith had decreased in diameter (2.5cm × 1.5cm) since the previous radiographs and the right uroliths were not evident anymore (Figure 14 and 15). Urinalysis showed still alkaline urine (pH 8), pyuria (75 WBC per HPF), haematuria (12 RBC per HPF) with bacteriuria 3+ and epithelial cells (greater than 20 per HPF). The culture grew a multiresistant Escherichia coli again. Treatment was maintained as previously.
Last time we rechecked the dog, it was one month after her last appointment (four months after initial presentation). She had much improved as her owners had been on holidays and, when they returned, even felt the dog was brighter than before. Clinical examination was unremarkable. Abdominal radiographs were repeated and showed the left kidney stone was only a quarter of the size compared to the previous radiographs, without any visible radiopaque stones in the right kidney (Figures 16 and 17). A urine sample brought by the owners showed still pyuria (40 WBC per HPF); however, not as marked as previously, with bacteriuria (Gram-negative rods and cocci) and a much more normal pH (6).
It is possible the dog initially developed calcium oxalate uroliths, as miniature schnauzers are predisposed to formation of these stone types. The uroliths could have led to a secondary UTI and struvite formation over the nidus of a calcium oxalate urolith. However, so far the calculi are still dissolving, so whether a nidus of oxalate calcium exists still remains unclear.
Struvite crystals are usually formed of 100% magnesium ammonium phosphate. In dogs, most of the struvite uroliths are secondary to UTIs with urease-producing bacteria, although some sterile struvite urolithiasis have been reported. The most common organisms associated with struvite crystal development in dogs are Staphylococcus species and, less commonly, Streptococcus, Proteus or Klebsiella species.
Only 1.4% of E coli organisms are urease-producing bacteria. These urease-producing bacteria have the ability to hydrolyse urea into ammonia, bicarbonate and carbonate. The ammonium, once formed, is ready to combine with phosphate and magnesium, which are common minerals in the urine, forming ammonium magnesium phosphate. The bicarbonate increases the urine pH, which makes the struvite crystals less soluble and urinary supersaturation of these ions occurs.
Medical dissolution of these uroliths requires a combination of antibiotics and a dissolution diet. The antibiotics choice is based on the results of the culture. In this case, antibiotics were initially chosen based on the results of the Gram stain (as the results of the culture were not initially present) and, also, as the dog had a septic peritonitis, a fluoroquinolone was chosen to reduce the risk of a septic endotoxaemia. Once the results of the culture were received, the antibiotics were maintained.
For long-term management of these patients, it is important to maintain antibiotic therapy at least four weeks after complete dissolution of the uroliths. This is due to the fact the bacteria can be trapped into the layering of the stones. Therefore, dissolution of fragmentation of these uroliths can lead to a spread of these bacteria in the urine, which can lead to reinfection if the antibiotics are no longer maintained.
In this case, Staphylococcus was first identified; however, consequently, a multiresistant E coli had been cultured, which is the most common bacteria causing UTIs in dogs. The same antibiotic choice was maintained, as most of the antibiotics tested for showed a degree of resistance and it was thought increasing the dose would increase the bactericidal effect and reduce likelihood of selecting resistant pathogens. At the last recheck, the pyuria had much improved since the previous recheck and the pH had normalised, probably reflecting an appropriate response to antibacterial treatment.
Antimicrobial treatment use in multiresistant infections is controversial. It could have been decided to use antibiotics not licensed for use in companion animals, such as chloramphenicol and aminoglycosides. However, for adequate dissolution of urinary calculi, antimicrobial therapy should be used until the calculi have been dissolved, which means, in this case, long-term antibiotics would have had to have been used that have potential serious side effects (such as bone marrow suppression or nephrotoxicity). The appropriate response to treatment and continued improvement in this case led to the decision of maintaining the antimicrobial therapy previously used.
A dissolution diet, in combination with the antibiotic, is always recommended, as antibiotics have little ability to penetrate struvite stones and stone dissolution will take significantly longer than without the addition of a diet. The diets have relatively low protein content (therefore reducing urea production), are low in calcium, magnesium and phosphorus, and are supplemented with sodium chloride to increase water intake. Wet diets are preferred as they also increase water intake, having a dilution effect in the urine and increasing the solubility of ammonium magnesium phosphate crystals.