28 May 2021
Lotfi El Bahri DVM, MSc, PhD, discusses the case of a Labrador that swallowed the contents of a tube it shouldn’t have in this latest from the occasional Case Notes series.

Image: © Viorel Sima / Adobe Stock
You are presented with a seven-month-old female Labrador retriever weighing 18.2kg at your emergency veterinary hospital.
The dog’s owner says he is applying a cream daily to treat proliferative skin diseases and has found his dog chewing the tube (40g) – the label of which listed the active ingredient as 5-fluorouracil (5-FU) 5%. The owner has noted rapidly thereafter profuse vomiting and a long-lasting seizure.
What is the toxic dose and mechanism of toxicity of 5-FU?
5-FU is a structural analogue of uracil (a fluorine atom at the C5 position in place of hydrogen) and thymine (5-methyluracil). Uracil, a pyrimidine nucleobase, is one of the four constituent nucleobases found in RNA. Thymine, also a pyrimidine nucleobase, is one of the four constituent nucleobases found in DNA. Many of the enzymes that participate in uracil or thymine metabolism also effectively metabolise 5-FU.
5-FU, an “antineoplastic” or “cytotoxic” agent, belongs to the class of fluoropyrimidines. It is administered by continuous IV infusion as chemotherapy across a variety of tumours and topically as a 5% cream to treat skin cancer in human medicine.
Poisoning can occur when a dog punctures a tube and ingests the topical preparations of 5-FU. Toxicosis can also occur when pets lick an owner who has applied the cream to himself or herself.
The US Food and Drug Administration is alerting veterinarians that dogs are at risk of death when exposed to the fluorouracil cream 5% intended for use in people. The minimum lethal oral dose in dogs is 20mg/kg; clinical signs of toxicosis are seen as low as 8.6mg/kg. Doses greater than or equal to 40mg/kg are reported to be fatal. The oral median lethal doses are 100mg/kg and 230mg/kg in rats and mice respectively.
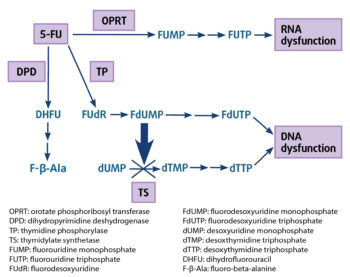
5-FU rapidly enters the cells using the same facilitated transmembrane carrier system as uracil. Mechanism of toxicity revolves around the multiple metabolism pathway of 5-FU, which is converted to several toxic metabolites by biochemical activation (Figure 1).
In addition, non-linear kinetics of 5-FU can lead to a high increase in plasma concentrations and toxicity. Several mechanisms of toxicity exist:
5-FU readily crosses the blood-brain barrier, and distributes into CSF and the brain, with high concentrations in the dog cerebellum. It is metabolised via a three-step enzymatic process – 85% by the primary enzyme dihydropyrimidine dehydrogenase to fluoro-beta-alanine (F-β-Ala). F-β-Ala causes neuropathological changes (vacuoles and necrosis/softening-like changes) in several areas of the cerebrum, responsible for the seizures.
In another pathway, 5-FU is metabolised in the liver to fluoroacetate, which enters the tricarboxylic acid (TCA) cycle in place of acetate and is converted to fluorocitrate, which competitively inhibits aconitase, thereby preventing the conversion of citrate to isocitrate, inhibiting the TCA cycle.
Inhibition of the TCA cycle (also called the Krebs cycle or citric acid cycle) by fluorocitrate blocks the gamma-aminobutyric acid (GABA) shunt, which produces GABA, the major inhibitory neurotransmitter in the CNS, contributing to the development of seizures.
The mechanism may be multifactorial. Fluoroacetate is responsible for the toxicity to cardiomyocytes. Direct toxicity also includes vascular endothelial thrombogenicity. Coronary spasm, protein kinase C-mediated, induces myocardial ischaemia. Impaired function of the heart’s left ventricule results in cardiogenic pulmonary oedema.
A small fraction of 5-FU (1% to 5%) is converted intracellularly to three main active metabolites – fluorodeoxyuridine monophosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP) and 5-fluorouridine triphosphate (FUTP; Figure 1):
FdUMP binds irreversibly to thymidylate synthetase to form a covalent stable complex, inhibiting this key enzyme required for the production of thymidine, thereby creating a thymine deficiency, which severely disrupts DNA synthesis and repair, resulting in lethal DNA damage and cytotoxicity.
FdUMP can be converted via a series of enzymatic steps to FdUTP, which in turn gets incorporated directly into DNA in place of thymidine, inducing double-strand DNA breaks and cytotoxicity.
FUTP is incorporated into all types of RNA instead of uridine triphosphate (UTP), producing a fraudulent RNA, and disrupts RNA processing and protein synthesis, and by blocking uracil. RNA-directed cytotoxicities are a major source of 5-FU toxicity symptoms.
These metabolites destroy cells dividing rapidly, such as epithelial cells of intestinal crypt and haematopoietic progenitor cells, resulting in severe gastrointestinal (GI) injury and bone marrow aplasia.
What are the main clinical features of 5-FU poisoning in dogs?
Clinical signs of 5-FU toxicosis can occur rapidly, usually within an hour, but can sometimes be delayed for up to five hours after ingestion. The GI signs first observed manifest as:
The neurotoxicity signs manifest as:
Death results of sudden cardiopulmonary arrest and can occur within six hours.
The bone marrow depression signs (occurs within 4 to 7 days and may last up to 30 days) is not commonly observed because most dogs with severe 5-FU poisoning do not survive the acute phase of toxicity.
5-FU is distributed in large amounts in bone marrow, destroying stem cells, resulting in bone marrow suppression. Bone marrow activity is decreased, resulting in abnormally low blood cell counts including red blood cells, white blood cells and thrombocytes (platelets).
Leukopenia and thrombocytopenia often occur around day five to seven, while anaemia is typically noted around day nine. ECG of a poisoned patient shows arrhythmias with Q-T interval prolongation (normal Q-T criteria: 0.15 sec to 0.25 sec in dogs). Prolongation of the Q-T interval is associated with high risk of ventricular arrhythmias and sudden cardiac death. Echocardiography reveals changes in left ventricular wall motion abnormalities.
Troponin I, biomarkers of myocardial damage, is increased (reference range in dogs less than 0.03ng/ml to 0.07ng/ml). Severe seizures cause rhabdomyolysis, causing marked increase in serum creatine kinase levels (reference range in dogs: 0IU/L to 190IU/L). The released myoglobin caused by muscle damage results in acute renal failure.

Blood urea nitrogen (BUN; reference range in dogs 2.5mmol/L to 9.6mmol/L) and serum creatinine (reference range: 44µmol/L to 159µmol/L) concentrations are elevated. Complete blood count may show anaemia (haemoglobin reference range 12g/dL to 18g/dL), neutrophilia (reference range 3,000 to 12,000 per microlitre/blood) and platelets (reference range 175,000 to 500,000 per microlitre/blood). Thrombocytopenia may cause bleeding diathesis.
How should 5-FU poisoning in dogs be managed?
5-FU poisoning is a life-threatening emergency. The specific antidote is uridine triacetate (UTA), a prodrug of uridine. Once absorbed in the bloodstream, UTA is rapidly deacetylated by non-specific esterases to uridine.
UTA delivers four to six times more uridine into systemic circulation than uridine itself. Circulating uridine is taken up into cells via specific nucleoside transporters and crosses the blood-brain barrier. Uridine is converted in the blood to UTP. UTA acts by augmenting intracellulary UTP levels, inhibiting competitively cell damage and cytotoxicity attributable to 5-FU incorporation into RNA.
The recommended dose in human medicine is 10g orally every six hours for 20 doses without regard to meals, starting as soon as possible within 96 hours after ingestion. UTA is not used in veterinary medicine due to being very expensive (US$74,000; £52,000).
An IV catheter should be placed. Benzodiazepines and barbiturates are not effective in managing seizures. Levetiracetam should be used: 30mg/kg IV over 5 to 15 minutes or 60mg/kg SC, or 40mg/kg rectally. In case of refractory seizures, administer propofol (3mg/kg to 6mg/kg IV initial bolus) followed by 0.1mg/kg/min to 0.6mg/kg/min constant rate infusion (CRI). Alternatively, 2% to 2.5% concentrations of isoflurane alone with oxygen can be used. For maintenance, use 1.5% to 1.8% concentrations of isoflurane in oxygen.
Attention to airway and breathing are paramount. Intubate affected animals and provide artificial respiration with O2 during convulsions. ECG and continuous cardiac monitoring should be performed. Correction of severe lactic acidosis (normal values in dogs: pH less than 7.33; standardised base excess less than -4mmol/L) by sodium bicarbonate 8.4% solution: 1ml/lb to 2.5ml/lb bodyweight CRI depending on the severity of the acidosis, over a four-hour period.
The induction of emesis is contraindicated. To prevent further absorption of 5-FU from the intestinal tract, use a single-dose activated charcoal (AC;1g/kg to 4g/kg) mixed with water to make a 20% slurry (1g/5ml water) via nasogastric tube as soon as possible post-ingestion, and after the airway is secured.
AC admitted orally is contraindicated in convulsing or comatose animals.
Maintain the patient on IV-balanced crystalloid fluids such as lactated Ringer’s solution 40ml/kg/hour to 90ml/kg/hour.
Severe vomiting should be treated by antiemetic serotonin antagonists such as ondansetron: 0.1mg/kg to 0.2mg/kg IV slow bolus every 8 to 12 hours. Metoclopramide is contraindicated (excitatory effects can occur).
Treat abdominal pain
µ agonist opioids should be administered: buprenorphine 2µg/kg/hour to 4µg/kg/hour CRI or 0.01mg/kg to 0.02mg/kg every four to eight hours.
Alternatively, use fentanyl 5µg/kg IV followed by 3µg/kg/hour to 5µg/kg/hour CRI.
Alternatively, use tramadol 4mg/kg to 6mg/kg every six hours IV or IM.
Administer loop diuretic: furosemide 2mg/kg to 4mg/kg IV. These large doses may need to be repeated (initially every one to two hours) until the respiratory rate and dyspnoea start to decline. Once stable, the dose should be reduced to 0.5mg/kg to 2mg/kg every 8 to 12 hours, as dictated by clinical status.
Assess BUN, creatinine and electrolytes within three days of initiating administration.
Thiazide diuretics (for example, chlorothiazide, chlorthalidone) are contraindicated (may impair clearance of 5-FU). Use a rapid‑acting positive inotropic agent: dobutamine 5µg/kg/min to 15µg/kg/min CRI diluted in 5% dextrose.
Filgrastim (a granulocyte colony stimulating factor; G-CSF), a 175 aminoacid protein, stimulates the bone marrow to increase production of white blood cells. Administer 5µg/kg to 15 micrograms/kg) SC once daily for seven days.
G-CSF is not species-specific. The attachment of a polyethylene glycol molecule to filgrastim (pegfilgrastim) extends the drug’s half-life, allowing for less-frequent dosing.
Destruction of the GI mucosal barrier and high potential for bacterial translocation, as well as leukopenia, may predispose the animal to secondary bacterial infections and sepsis. Administer broad-spectrum antibiotics such as fluoroquinolones: enrofloxacin 10mg/kg to 15mg/kg IV every 24 hours or aminoglycoside antibiotics such as gentamicin 6.6mg/kg IV every 24 hours. Metronidazole is contraindicated (CNS toxicity).
Supportive treatment and monitoring is important for a minimum of one week during the phase of acute toxicity, and thereafter four weeks until bone marrow function returns to normal, if the patient survives.
Owners should be advised to store all medications safely out of the reach of pets. Safety discards any cloth or applicator that may retain 5-FU medication and avoid leaving any residues of the medication on hands, clothing, carpeting or furniture. Evaluation of persistence, bioaccumulation and toxicity properties supports the conclusion of no significant environmental risk for 5-FU and/or their metabolites.