14 Mar 2017
Miguel Martinez highlights considerations when dealing with anaesthetised intracranial disease patients.

Intracranial disease and its associated clinical neurological signs are relatively common in dogs and cats, with increased incidence in middle-aged and old individuals.
To achieve an accurate diagnosis, performing advanced imaging techniques, such as MRI, that require the patient to be anaesthetised is often indicated. Anaesthesia is also required in those cases where therapeutic options include craniotomy and/or radiotherapy. The anaesthetic risk is understandably higher than average and numerous specific considerations have to be taken into account (Figures 1 and 2).
A thorough evaluation of the patient’s health status and its interaction with the anaesthetic/surgical procedure is essential to optimise outcomes. This is called “anaesthetic considerations”. It is recommended to perform an exhaustive physical exam with particular emphasis on the cardiovascular system and CNS. For example, little clues, such as decreased mentation and slow heart rates (increased vagal tone), can be an indication for a lifesaving intervention to manage raised intracranial pressure.
Generally speaking, pathology affecting the brain stem – from where all the basic functions of the body are controlled – are associated with alterations in the level of alertness, cardiac rate, and rhythm abnormalities and abnormal respiratory patterns. These are very high risk anaesthetic candidates. Conversely, disease affecting the cortex is more likely to cause seizures and abnormal behaviour, but anaesthetic management is usually less complicated, albeit still potentially challenging (Panel 1).
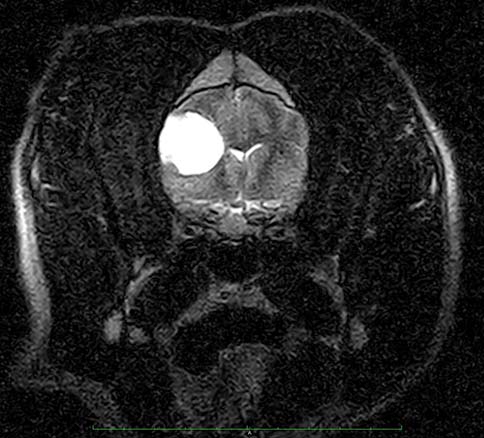
A comprehensive blood analysis – including haematology, biochemistry and electrolytes – is always indicated to rule out metabolic causes of neurological signs (such as portosystemic shunt, insulinoma, neurogenic diabetes insipidus and hypoadrenocorticism). It also helps assess other concomitant problems that may increase the risk of anaesthetic morbidity (complications) and mortality. The author would like to stress the importance of measuring glucose before anaesthetic in these patients. It is a simple and inexpensive test, but, nevertheless, a “must do”.
Hypoglycaemia must be treated promptly to prevent brain damage, and the aetiology investigated. For example, a low glucose reading can lead to a diagnosis of insulinoma (confirmed through simultaneous plasma insulin measurement and abdominal ultrasound), which would prevent unnecessary anaesthetic for MRI (Figure 3).
It is also part of the pre-anaesthetic assessment to list drugs the patient takes and evaluate the impact they can have in the procedure. Antiepileptic drugs – such as imepitoin, phenobarbital, potassium bromide and levetiracetam – and analgesic drugs, such as NSAIDs, corticosteroids and opioids, are often prescribed. The use of these drugs is often beneficial for the patient. It must be emphasised a correct preparation of the patient before the anaesthetic reduces greatly mortality and morbidity and, even in the most urgent cases, it is good to carefully assess and stabilise the patient before embarking on procedures that require an anaesthetic. In summary, take time to think before you take the next step.
Cardiovascular
Neurological
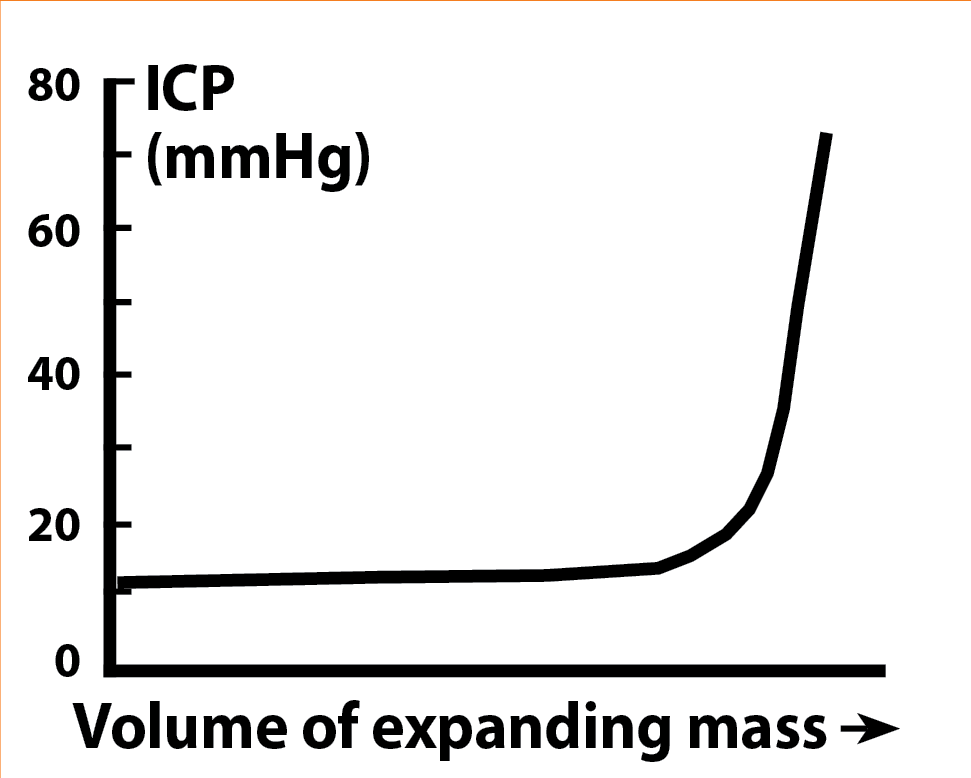
The main challenge for the anaesthetist in these cases is to maintain and optimise the delicate balance between intracranial pressure and cerebral perfusion. The cranium – a non-distensible structure – contains the encephalon, its blood supply and the cerebrospinal fluid (CSF). Any increase in the volume of tissue or fluid in the skull will automatically be translated into an increase in intracranial pressure.
Initially, growth of any intracranial mass is compensated by means of evacuating CSF, which maintains the intracranial volume stable. However, once this mechanism is exhausted, any small increase in intracranial volume will cause a drastic increase in intracranial pressure (Figure 4). As a direct consequence, a drop in cerebral blood flow (CBF) will occur (oxygen and glucose delivery
are compromised). The end result is severe neuronal damage since the brain has a high metabolic rate and little functional reserve.
In normal conditions, CBF is regulated by local factors (oxygen and glucose metabolism). If the metabolic needs are not met, cerebral vasodilation occurs to increase CBF, and improve oxygen and glucose delivery. The main triggers of cerebral vasodilation are hypoxaemia and hypercapnia. The opposite scenario (hyperoxaemia and hypocapnia) would cause vasoconstriction. Vasodilation and vasoconstriction alter intracranial blood volume and, therefore, affect the pressure-volume-flow equilibrium.
Cerebral perfusion pressure (CPP) is determined by the formula CPP = MAP − ICP. (MAP = mean arterial pressure. ICP = intracranial pressure).
It is important to understand pressure and flow are not equivalent or interchangeable concepts. A high pressure does not guarantee adequate blood flow (for example, severe vasoconstriction). However, we can assume, in the majority of cases, a CPP below 50mmHg does not allow a sufficient CBF. In a healthy brain, autoregulation helps maintain CPP greater than 50mmHg, providing MAP is in the range of 50mmHg to 150mmHg, by adjusting local systemic vascular resistance. Unfortunately, severe intracranial disease (for example, a brain tumour) disables autoregulation and CPP is directly reliant on MAP-ICP. Therefore, if MAP decreases or ICP increases dramatically, CPP suffers and brain damage may happen.
So, how do we apply these concepts to a clinical case? In practical terms, a dog or cat suffering from increased ICP (for example, a brain tumour) needs to be anaesthetised for diagnostic or therapeutic reasons. Our anaesthetic aims are to:
Hyperosmotic fluids, such as hypertonic saline 7.2% or mannitol, can be used before or during the anaesthetic to reduce interstitial oedema and improve CBF. They draw fluid from the tissue into the circulatory system. Hypertonic saline must be administered as a slow bolus of 2ml/kg to 4ml/kg over 5 to 10 minutes. It improves intravascular volume and myocardial contractility, can cause arrhythmias if injected too fast and is contraindicated in dehydrated animals. It is usually followed by isotonic fluids at moderate rates.
Mannitol is a polysaccharide dissolved in pure water – usually at concentrations of 10% to 20%. It can precipitate at room temperature, but the crystals can dissolve if the solution is warmed up. Administered as a bolus over 20 to 30 minutes, it causes an initial increase in circulating volume, but, eventually, can cause hypovolaemia due to osmotic diuresis. This has to be taken into account and the volume loss needs to be replaced after with crystalloid solutions. It improves CBF in a similar way to hypertonic saline and is also an oxygen radical scavenger.
In patients with an intracranial mass, a general consensus exists to recommend maintenance of MAP above 80mmHg to make sure CPP is above 50mmHg. As an example, if a dog has an ICP of 20mmHg (normal between 2mmHg to 10mmHg), from the previous equation CPP = MAP – ICP we know a MAP of at least 70mmHg is required to remain above the minimum acceptable CPP required; therefore, the recommendation of 80mmHg to have a “safety cushion”. MAP is maintained by means of titration of anaesthetic agent (remember, most anaesthetics cause dose-dependent vasodilation), optimisation of circulating blood volume and judicious use of vasopressors, if required (Figures 5 and 6).
In anaesthetised patients, another tool to regulate intracranial volume is to control end-tidal CO2 (ETCO2), usually applying mechanical ventilation. Cerebral vascular resistance is extremely sensitive to local concentration of CO2. A decrease in CO2 will cause vasoconstriction and reduce the total amount of water within the cranium, and the opposite is true for increases in carbon dioxide. This CO2 responsiveness is extremely fast and can be lifesaving in emergency treatment of severe intracranial hypertension. The general consensus is to maintain ETCO2 around 30mmHg to 35mmHg in anaesthetised patients. Sustained hypocapnia below 30mmHg reduces CBF and can be detrimental (Figure 7).
In preparation for surgical excision of brain tumours in dogs and cats, oral corticosteroids are often prescribed. They are thought to reduce vasogenic oedema around the mass, which, in turn, reduces intracranial volume and results in marked – albeit transient – clinical improvement. Anticonvulsants are also recommended if seizures are present. They usually have minor interactions with general anaesthetics and are neuroprotective.
The target organ of anaesthetic agents is the brain and, by extension, the CNS. Because of the very nature of intracranial disease, the effect of anaesthetic drugs is less predictable in this patient population. A disruption of the blood-brain barrier is often present and, as a result, standard doses of anaesthetics can cause extremely marked effects. We must always exert extreme caution when we administer anaesthetic drugs in these patients, starting with low doses and titration to effect, if necessary. Where possible, short-acting and reversible drugs (available antagonist) should be used.
Premedication with sedatives and opioids is commonplace in veterinary anaesthesia. It allows handling of the animal in a stress-free fashion and reduces the dose of anaesthetic agents required. However, if the patient is already subdued – or even obtunded – it may not be advantageous. Placement of an IV catheter and administration of fluids, such as hypertonic saline and mannitol, may be all that is required before induction of anaesthesia.
If premedication is indicated, the author recommends short-acting agents – such as pethidine, butorphanol or dexmedetomidine – with the advantage they can all be antagonised. They can be used alone or in combination. As a tip, the more subdued the patient, the lower the dose of sedative used.
The author often uses low doses of dexmedetomidine (1µg/kg to 2µg/kg) or medetomidine (2µg/kg to 5µg/kg) IV in less severely affected animals. It provides excellent sedation, marked anaesthetic sparing effects and reliable cardiovascular stability. Bradycardia has to be seen as a common, but acceptable, side effect.
Benzodiazepines, such as midazolam and diazepam, can be used safely, causing mild cardiovascular effects and moderate anaesthetic sparing effect. They are, nonetheless, mild sedatives that occasionally can cause excitation, which is counterproductive. They are more reliable in combination with opioids.
Acepromazine is not recommended due to long duration of action, lack of specific antagonist and profound vasodilation, leading to hypotension.
Induction of anaesthesia can be achieved with IV agents, such as propofol or alfaxalone. They both have similar clinical effects and can be safely used titrated to effect. The aim of induction is to enable endotracheal intubation. It is beneficial to minimise the response to laryngeal stimulation (coughing) that can cause a transient increase in intracranial pressure. Some anaesthetists advocate the use of IV lidocaine (2mg/kg) as a coinduction agent to reduce this response. Topical lidocaine has also been advocated.
Maintenance of anaesthesia can be continued with a constant rate infusion (CRI) of propofol (or alfaxalone) or inhalant anaesthetics. Many human studies and some veterinary studies recommend propofol for maintenance of anaesthesia in individuals with intracranial disease due to its neuroprotective properties. Propofol reduces cerebral metabolism and preserves cerebral vascular autoregulation. Recovery after interruption of the infusion is usually quick.
Inhalant anaesthetics can also be used for the maintenance of anaesthesia. Sevoflurane in dogs is more desirable than isoflurane. It preserves cerebral autoregulation and normotension better than isoflurane at equipotent doses. Sevoflurane allows rapid recoveries and permits early neurological assessment postoperatively. Isoflurane at low doses (below 1 × minimum alveolar concentration) is also acceptable.
As already discussed, the main targets during the procedure are to maintain ETCO2 below 35mmHg and MAP greater than 80mmHg. It is also advantageous to avoid marked changes in arterial blood pressure. To this effect, it is important to provide adequate intraoperative analgesia – usually by means of a pure µ opioid agonist, such as methadone or fentanyl in dogs. The author also advocates the use of a CRI of dexmedetomidine or medetomidine. These alpha-2 agonist drugs can also be used as part of the pre-anaesthetic medication. They are excellent sedatives and analgesics, and contribute to a balanced anaesthetic by markedly reducing the amount of induction and maintenance anaesthetic required.
Besides, they have a sympatholytic effect providing a stable haemodynamic pattern (no swings on arterial pressure and heart rate) during endotracheal intubation, surgical stimulation and postanaesthetic recovery. In combination with opioids, they provide excellent analgesia, but also more pronounced side effects, such as hypoventilation and bradycardia.
Hypoventilation is irrelevant, since the patient must be mechanically ventilated. Bradycardia can be treated pharmacologically if clinically detrimental. Alpha-2 agonists can induce vomiting, but this complication is rare when low doses are administered by the IV route. Since many of these patients receive corticosteroids, use of NSAIDs is contraindicated. In dogs, the author has often used paracetamol as an adjuvant analgesic. Paracetamol is a cyclo-oxygenase 3 (COX-3) inhibitor.
COX-3 is an enzyme involved in the development of inflammation and pain. It is present in high concentrations in the brain of dogs and rats. Paracetamol provides analgesia by blocking this and other pain pathways. Paracetamol is contraindicated in cats due to severe toxicity at clinical doses.
Recovery can be a tricky phase. It is important to provide a smooth transition between unconsciousness and fully awake. The animal has to be weaned off the ventilator, and a regular and effective breathing pattern regained. Remember, hypoventilation and hypercapnia cause vasodilation and must be avoided as it can lead to a dangerous increase in ICP.
The patient must be placed in a quiet area with intensive care facilities. It is advisable to prepare all monitoring and support equipment in advance to prevent accidents and stress in the early stages of recovery. Blood pressure, ECG and peripheral oxygen saturation (SpO2) must be monitored. The endotracheal tube can be removed as soon as the swallowing reflex returns. It is very important to prevent excitation, coughing and vomiting at this point. Oxygen has to be supplemented if SpO2 is less than 92%. In some cases, it can be useful to maintain a light level of sedation for a short period of time to allow gradual elimination of the anaesthetic agents. Either dexmedetomidine or propofol can be used to this effect (Figures 8 and 9).
Typical complications are hypothermia, slow recovery, bradyarrhythmias, apnoea and cardiac arrest – often due to intracranial hypertension. The initial management has previously been discussed. In some cases, it can be necessary to perform a craniotomy to decompress the brain (for example, blood clots).
After craniotomy analgesia can be continued with paracetamol and an opioid taking care not to cause excessive respiratory depression. Buprenorphine at 0.01mg/kg to 0.02mg/kg IV has proven to be safe and effective in dogs and cats, but many other alternatives are available. The level of care and monitoring can be gradually stepped down as the patient regains full consciousness, ambulation, and eating and drinking well. However, a minimum of at least 24 hours of intensive care is recommended.
Seizures are not uncommon perioperatively and must be treated promptly. Some protocols include antiepileptic drugs pre-emptively (phenobarbital 2mg/kg twice a day in dogs); however, the author prefers to assess this individually based on previous clinical signs and likelihood of occurrence. The case in Panel 2 describes an anaesthetic protocol used in dogs undergoing craniotomy. It was presented at the 2012 World Congress of Veterinary Anaesthesia in Cape Town, South Africa.
The anaesthetic protocol described provides excellent haemodynamic stability. Recoveries are rapid and smooth, allowing early neurological assessment after surgery. Other factors, such as tumour location and surgeon skill, may be better predictors of peri-anaesthetic complications.
The anaesthetic records of 13 dogs subject to craniotomy for brain tumour removal were retrospectively analysed.
Owner consent for the procedure was obtained. Prednisolone and phenobarbital were administered for several days before the procedure. The dogs – seven males and six females aged (mean plus or minus standard deviation) eight plus or minus three years, weighing 29kg plus or minus 13kg and American Society of Anesthesiologists (ASA) health status 2 to 4 – were premedicated with IV medetomidine and methadone, followed by IV propofol to effect, to allow endotracheal intubation. Anaesthesia was maintained with sevoflurane in 100% O2 and an IV infusion of medetomidine. Some individuals received fentanyl, methadone or atracurium at the discretion of the anaesthetist. All dogs were mechanically ventilated during the surgical procedure to maintain ETCO2 less than 5.3 kilopascals (kPa; 40mmHg). Fluid therapy consisted of saline 0.9%.
Heart rate (HR), respiratory rate (fR), blood pressure, end-expired carbon dioxide tension (PE’CO2), end-tidal concentration of sevoflurane (PE’SEVO), SpO2 and temperature were monitored. Postoperative analgesia consisted of IV paracetamol and buprenorphine.
All dogs were discharged from the hospital. Anaesthetic and surgical time was 174 plus or minus 30 minutes and 112 plus or minus 31 minutes, respectively. The doses of anaesthetics administered were as follows:
The values of the variables monitored were:
Postanaesthetic temperature was 38°C plus or minus 0.9°C, time to extubation was (median, range) 10, 5 to 63 minutes and time to sternal recumbency 19, 15 to 78 minutes. The most frequent complications were moderate haemorrhage and bradycardia.