26 Aug 2022
Ian Ramsey BVSc, PhD, DSAM, DipECVIM-CA, FHEA, FRCVS covers developments in the methods for which veterinarians and owners can keep track of this disease in dogs.

Cataracts are a sign of failure to rapidly stabilise diabetes mellitus. Not all can be prevented, but we must try harder with our owners to achieve better and quicker stabilisation.
Diabetic monitoring in dogs is undergoing a revolution. The introduction of economically viable methods of measuring interstitial glucose has changed our ability to closely monitor glycaemic control over several days, and adjust insulin doses, formulations and feeding practices to achieve the best glycaemic control possible.
The introduction of reliable and economic methods to measure glycosylated haemoglobin has changed our ability to assess average glycaemic control over a clinically relevant time span for diabetic rechecks.
These monitoring methods, when taken together, are set to change our management of diabetes in veterinary clinical practice. This article will briefly review these developments while emphasising the need for effective home monitoring and regular clinical assessment of diabetic dogs.
Diabetic dogs suffer from several complications caused by unstable diabetes that negatively impact on the animal’s quality of life and the owner-animal bond.
These include the (often rapid) development of cataracts, persistent polyuria, lethargy and difficulty maintaining weight.
On top of this, the increased costs and worry of managing an unstable diabetic dog may deter owners from continuing with treatment – especially if it is perceived that such treatment has not restored the dog back to the good health it enjoyed before the diabetes developed.
Cataracts are one of the most common and serious complications of diabetes in dogs, and data suggests they have a much higher risk of developing cataracts than non-diabetic dogs (Yoon et al, 2020).
In one study, 12% of diabetic dogs had cataracts at the time of diagnosis and within one year 75% had developed them (Beam et al, 1999). Most of these cases reflect a failure to recognise and treat diabetes effectively in the early stages, although other factors may have been involved in cataract formation.
Early and effective stabilisation of diabetes followed by effective monitoring to ensure stabilisation is maintained is likely to play a key role in cataract prevention.
Although owners also have responsibility for this situation, the veterinary profession has an important role in trying to stabilise dogs faster and more effectively to prevent this complication.
Phacoemulsification is an effective method of removing cataracts and restoring vision in such cases, but it is not without its complications and significant costs. Many owners cannot afford phacoemulsification, so prevention must be a priority.
Many dogs who do have the surgery still require topical eye medications for sustained periods. Aldose reductase inhibitors may slow the progression of cataracts and one has provisionally been approved in the US, but these only slow development and are no substitute for effective diabetic stabilisation.
Equally, once the dog has developed cataracts, little point exists in trying to achieve higher levels of stabilisation as the cataracts will not resolve. The time to get diabetics properly stable is before the cataracts start to develop.
Diabetic dogs in the UK that are treated in primary care practice have a poor median survival time compared to cohorts treated in referral practices (Cartwright et al, 2019; Mattin et al, 2014). Some of this difference may be due to failure of early stabilisation as those dogs that were stabilised quickly survived significantly longer in one study (Cartwright et al, 2019).
Before we consider how we stabilise diabetic dogs, it is useful to define what is our goal.
The author defines stable diabetic dogs to his clients as those dogs that show no clinical signs of diabetes, whereas unstable diabetics show one or more signs of diabetes despite insulin administration.
Note that this definition does not include any assessment of biochemical markers of diabetes, such as measurements of fructosamines, urine, blood glucose and so forth. These are only tools clinicians and owners can use to try to adjust the diabetic management to achieve stability. Fructosamines, blood glucose curves, urine tests and so forth are not the end result: they are the means to achieve the end result.
Therefore, it follows that a dog that (truly) has no clinical signs of diabetes does not need to have its insulin or any other aspect of its diabetic management changed because of any biochemical result.
If the premise is accepted that the assessment of diabetic stability is overwhelmingly a clinical concept, and not a clinic-pathological one, then a lot of time and money can be saved in some cases.
The problem is not all signs of diabetes are immediately obvious to owners or clinicians. Mild hyperglycaemia may produce few obvious clinical signs, but still have significant clinical impact. Specifically, in dogs, the development of diabetic cataracts may occur in the absence of other, more obvious, clinical signs. Therefore, work to define the prognostic value of various laboratory tests is important.
Diabetics that are treated with insulin are not normal dogs. They have no ability to adjust their insulin concentrations, and so a strict control of the diet and scrupulous attention to health are important.
The most important aspect of diet is palatability: if the dog does not love the food, then no point exists feeding it.
The second most important is energy intake: obese dogs need to be dieted and dogs that are thin need to gain weight as a key clinical target. Thereafter, concepts such as the balance of complex carbohydrates to fat and protein, and avoidance of simple sugars need to be considered, but never forgetting the first two aspects.
The starting dose of insulin used by the author is 0.5IU/kg of lente insulin twice daily. Protamine zinc insulin (PZI) is also available for use in dogs at a starting dose of 0.5IU/kg once daily (the author uses PZI as first choice for cats). Insulin should be adjusted every week in an unstable diabetic dog.
Initially, single unit changes (up to doses of 10 units) are used then in two-unit changes. Insulin syringes are not reliably accurate at 0.5IU increments and many owners struggle to measure this, so such doses should be avoided if possible. Small doses of insulin are particularly difficult to measure using insulin syringes, and insulin pens are likely to be more accurate (Malerba et al, 2021). Most dogs need about 1IU/kg (give or take 0.5IU/kg) per dose to achieve stability.
Entire female diabetic dogs cannot be stabilised for any relevant period until they are surgically neutered. This should be done as soon as possible (as it may even cure them of the diabetes), and is a medically necessary procedure that should be treated as an urgent surgical procedure.
Only if a dog is ketotic or ketoacidotic should the procedure be delayed: proper stabilisation is not required (and may not even be possible anyway).
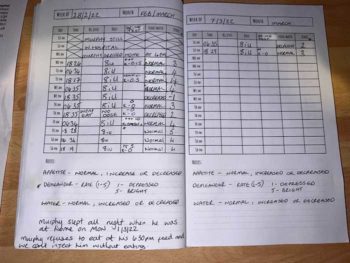
A key requirement of any protocol of diabetic stabilisation is regular home monitoring of weight, appetite, thirst, urine production and the presence of glucose/ketones in the urine. Some owners may struggle to monitor all of these, but anything is better than nothing.
The author gets the owners of nearly all of his diabetic dogs to monitor urine on a daily basis. Those that are clinically stable usually have, on average, a trace of glucose in the urine (but this may vary from none to significant glucosuria from day to day).
Dogs with persistent moderate or marked glucosuria will be polyuric (by osmosis) and, therefore, are not stable. Veterinary assistance in the assessment of these records is vital in the early stages, but later owners usually become more confident at identifying issues and actions arising from these.
The author does not encourage owners to perform blood glucose curves at home, preferring continuous glucose monitoring systems.
Following the diagnosis of diabetes, a period of instability is to be expected while the correct insulin dose, formulation and diet are found.
During this time, underlying diseases that may contribute to instability (such as cystitis, pancreatitis, and other endocrine diseases) need to be identified and treated as best as possible.
Some of these underlying diseases may require expensive investigations, but many can be easily identified and treated. For example, the presence of significant dental disease with purulent gingivitis and pain may have a profound effect on diabetic stability, and can be treated alongside the diabetic stabilisation.
Experimentally induced diabetes in dogs is usually stabilised within a month, and in one major study the median time to stabilise dogs using lente insulin was 35 days (Monroe et al, 2005). Assuming that these underlying diseases can be managed relatively quickly, it should take not more than two months to stabilise a naturally occurring case of diabetes in a dog.
Failure to achieve stabilisation within this timeframe should cause a review of the case and the protocols being used to stabilise it.
Many textbooks and journal articles have described diabetic stabilisation using blood glucose curves to illustrate some of the issues encountered. From here has grown the concept of using blood glucose curves to achieve stabilisation.
However, blood glucose curves in stable diabetic dogs are known to show considerable day-to-day variation (Fleeman and Rand, 2003).
Such is the variation that one day the curve may suggest a dose increase and the next day a dose increase (and yet the dogs are stable and, therefore, require no dose change).
A similar study has not been performed in unstable diabetic dogs, but data from continuous glucose monitoring systems would suggest similar results would be likely.
Furthermore, blood glucose curves are usually performed using glucometers that are themselves subject to a degree of variation compared to standard laboratory measurements.
Performing blood glucose curves in an hospital environment, with all its stresses and strains and artificiality, adds a considerably larger set of variable factors to an already unreliable set of measurements.
Finally, even among experts, little agreement can be found in the interpretation of curves.
So, blood glucose curves are a test where we cannot be sure of the results. Its results may vary from the normal situation and we are not sure of its interpretation. Is this really a “gold standard” method of stabilisation?
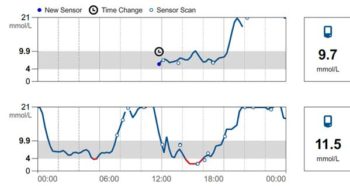
The newer alternatives to blood glucose curves are continuous glucose monitoring systems that measure interstitial fluid glucose. Early models needed regular calibration and were expensive, inconvenient, or unreliable.
However, the development of a flash glucose monitoring system (FGMS) that required no calibration marked a turning point for human and canine diabetics.
These systems are cheaper than blood glucose curves, produce more data over a longer period (and so provide an “average curve”) and, as they are used at home, are not influenced by the stress of hospitalisation.
These systems have been the subject of a range of scientific papers in recent years.
The major study in dogs found the devices easy to use, and documented a good correlation between the interstitial and blood glucose concentrations (Corradini et al, 2016). The FGMS was 93%, 99% and 99% accurate at low, normal and high blood glucose concentrations.
Mean standard deviation difference from the reference method was 0.12mmol/L (give or take 2.6mmol/L).
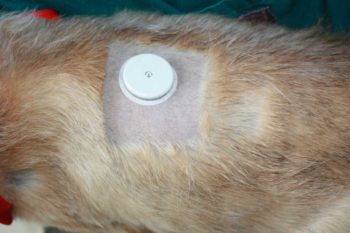
The future for wearable technology in canine diabetes is an exciting one. An implantable device that allows glucose monitoring for up to 180 days has been approved for use in humans in Europe and an initial trial on three dogs was successful (Tardo et al, 2022).
The sensor, not much bigger than a conventional microchip, is placed subcutaneously under sedation and local anaesthetic.
After the sensor has been placed, an external transmitter is stuck to the overlying skin for periods of 24 to 72 hours before it needs to be removed, recharged and reapplied.
The transmitter links to a mobile phone and provides a near continuous glucose monitoring for the life of the sensor.
This specific device may not catch on, but it shows what is coming in the future.
Fructosamine measurements have been available for dogs since the 1990s and are regularly used by many vets to guide the assessment of stability.
Increased fructosamines are found in most diabetic dogs and a tendency exists to see very high levels in unstable diabetic dogs (who would be expected to be showing clinical signs anyway).
However, fructosamines have not been regularly used in human medicine for many years because they are not reliable enough, as they are affected by other things apart from the average glucose concentration. They also only provide an assessment of stability over a relatively short time.
Fructosamines are not predictive of diabetic instability (and in humans, are not prognostic for the complications of diabetes).
A dog may have a very high concentration of fructosamines and still have few or only mild signs of diabetes mellitus; equally, very unstable dogs may have fructosamines that are only moderately increased.
In humans, the standard measure of long-term glycaemic control is haemoglobin A1c (HbA1c). This is formed from the reaction of haemoglobin with glucose in a manner analogous to the process that forms fructosamines from serum proteins. Molecules of haemoglobin last longer in the circulation than those of serum proteins, so haemoglobin A1c concentrations reflect longer-term glycaemic control (probably about two to three months in the dog).
In addition, in humans, HbA1c is less affected by other parameters (such as fever) than fructosamines (the notable exception being anaemias, particularly those with a reduced erythrocyte life span).
HbA1c levels are also known to be prognostic for the development of the complications of diabetes in humans.
A human with high levels of HbA1c is more likely to suffer from retinal, vascular and renal complications of diabetes. Changes to diabetic protocols (such as changes to insulin type) can be assessed in terms of their effect on average HbA1c concentration of humans treated with the new protocols.
By extrapolation from human medicine, it is reasonable to ask if measuring HbA1c in dogs could provide veterinary clinicians with a better measure of long-term glycaemic control.
Several studies have already been conducted on measuring haemoglobin A1c in dogs, but a reliable way of measuring it in the veterinary clinic has proved elusive, often because the methods that were used in humans proved impractical in dogs.
However, the development of immunoturbimetric assays for measuring HbA1c in humans have provided vets with a new opportunity (Goemans et al, 2017). These assays work well in dogs as they share a very similar amino acid sequence to humans at a critical area in their haemoglobin.
Sadly, cats do not have the same amino acid sequence in this region and so the immunological route does not work. One paper has already looked at one type of immunoturbimetric assay, but did not find good correlation with clinical status using fructosamine or haemoglobin A1c (del Baldo et al, 2020).

Experiences in the author’s own clinic using a different type of immunoturbimetric assay do not seem to agree with this paper, but further work funded by BSAVA PetSavers is ongoing.
The author’s hypothesis is that glycosylated haemoglobin will provide vets with an assessment of how well our diabetic protocols are actually performing over sustained periods and are likely to be of prognostic value.
Much work remains to be done and the author would be delighted to discuss individual cases with vets that have had HbA1c measured.
A chromatographic method of measuring haemoglobin A1c in dogs has been released on to the veterinary market in the US and samples can be sent from the UK, providing they are accompanied by proper documentation.
A study using this technique showed the haemoglobin A1c was more consistent with clinical evaluation of diabetes control than the serum fructosamine concentration (Norris and Schermerhorn, 2022).
This assay may also work in cats, but the results have not been published so far.
All researchers agree that using haemoglobin A1c (or fructosamine) as a stand alone assessment of diabetic stability is fraught with problems. These assays should be used alongside clinical findings and the owners’ home monitoring records as an additional check.
The assays also do not tell you why an animal is unstable – merely that is more or less likely to be stable, and more or less likely to suffer from complications in the future.
Increasing insulin doses in response to increased haemoglobin A1c (or fructosamine) may not be an appropriate response: some dogs may need a different type of insulin, different feeding patterns or treatment of a concurrent infection.
Simply increasing insulin doses in these circumstances risks the development of hypoglycaemia.
The management tools available to vets today to monitor diabetics are considerably better than they were even five years ago.
However, the “old school” methods that use owner records, urine glucose testing and regular weighing have much to commend them in the early stages of diabetic stabilisation.