28 Nov 2023
Luca Ferasin and Heidi Ferasin explore causes, diagnosis and management of these episodes which can cause great distress to owners.

A dual-chamber pacemaker, implanted to treat bradycardia and the associated clinical signs.
Fainting (or syncope) describes a transient, unprovoked and self-limiting loss of consciousness, usually leading to postural failure (falling) due to cerebral global hypoperfusion.
The onset of fainting is usually very rapid and these episodes tend to last for only a few seconds, although to the observer the affected subject may appear “dead” during the event. However, the subsequent recovery is spontaneous, complete and often uneventful.
Syncope, therefore, obviously differs from “sudden death”, which could be defined as an “irreversible fainting”. Therefore, the only difference between fainting and sudden death is that in one, you wake up.
In human medicine, the Multidisciplinary Task Force on Syncope introduced the term “transient loss of consciousness” (TLOC) to describe the wider concept of brief episodes of unconsciousness, rendering fainting as just one form of TLOC under the resulting classification scheme.
However, irrespective of the precise underlying cause of fainting, a sudden cessation of cerebral blood flow for six to eight seconds and/or a decrease in systolic blood pressure to 60mmHg has been shown to be sufficient to cause complete loss of consciousness in people. Critical physiological values that can induce fainting in dogs and cats are not available, but it is not unrealistic to believe they may be similar to those reported in human patients.
Fainting episodes pose a real diagnostic challenge, as the causes are diverse, carry vastly different risks and prognoses, and span various specialities. The use of an inconsistent terminology contributes to the confusion. For example, the term “collapse” is often used, inaccurately, in veterinary medicine to describe a fainting episode. Indeed, the word “collapse” derives from the Latin word collapsus, past participle of collabi, meaning “to fall apart”.
In human medicine, the term collapse defines “a state of extreme prostration and depression” or “a falling together of the walls of a structure” or “the failure of a physiologic system”. Therefore, for a purely semantic convention, we should probably limit the use of the word “collapse” to describe an instance of complete or partial occlusion of the lumen or cavity of an anatomical structure, such as in “tracheobronchial collapse” associated with tracheobronchomalacia or “circulatory collapse” observed in the cardiogenic shock.
Therefore, the authors prefer to use the simple and more familiar term “fainting” to describe any transient loss of consciousness, with the term “falling” used in cases where the patient loses postural control but remains conscious However, “falling” often accompanies a “fainting” episode, and in these circumstances, the “falling” episode is of pivotal importance since these falls can lead to injuries, hence the wider term “falling and fainting”.
Fainting is caused by cerebral hypoperfusion and is divided into neurally mediated syncope (reflex syncope), fainting due to orthostatic hypotension, and cardiac syncope (arrhythmic or associated with structural cardiovascular disease). The other major groups of TLOC are generalised epileptic seizures, functional TLOC (psychogenic TLOC mimicking either epilepsy or syncope), and a further group of miscellaneous disorders.
In dogs and cats, fainting is often associated with primary cardiovascular conditions, although some non-cardiovascular abnormalities need to be considered as differential diagnoses. However, orthostatic, metabolic and psychological causes of syncope, which represent common causes of fainting in people, are not typically described in veterinary medicine.
Iatrogenic arterial dilation may occur after administration of drugs that induce vasodilation, such as nitroprusside, acepromazine or amlodipine. All the abnormalities previously listed can potentially induce cerebral hypoperfusion and, ultimately, loss of consciousness.
In dogs and cats, the most common causes of fainting have been associated with prolonged periods of bradycardia (sick sinus syndrome, atrioventricular [AV] block, and atrial standstill) or tachycardia (paroxysmal ventricular tachycardia [VT] or supraventricular tachycardia [SVT]).
Other fainting events can be associated with myocardial disorders, left and right outflow obstructions, pericardial effusion, increases in intrathoracic and intra-abdominal pressure with subsequent reduced venous return during coughing, defecation, micturition, and swallowing (situational syncope) and congenital cardiac defects. Vasovagal (neurocardiogenic) fainting, which is one of the most common causes of fainting in people, is also occasionally observed in dogs and cats. A summary of potential causes of syncope is reported in Table 1.
| Table 1. Major causes of falling and fainting in people, dogs and cats. Aetiologies reported in italics are mainly observed in people | ||
|---|---|---|
| Cardiac | Vascular | Non-cardiovascular |
| Anatomical outflow obstruction (severe subaortic stenosis or pulmonic stenosis, heart base or intracardiac tumours) | Orthostatic hypotension (venous pooling = frequent in people, but not reported in pets) | Neurological (seizure disorders, head trauma, hepatic encephalopathy, narcolepsy, episodic falling in the cavalier King Charles spaniel, autonomic neuropathy) |
| Cardiac tamponade (pericardial effusion) | Pulmonary thromboembolism | Metabolic (hypoglycaemia) |
| Obstructive hypertrophic cardiomyopathy | Pulmonary hypertension | Psychological (anxiety, panic, somatisation disorders) |
| Myocardial ischaemia | Neurocardiogenic (vasovagal) | Iatrogenic (acepromazine, hydralazine, amlodipine, nitrates, and so forth) |
| Bradycardia (high-degree atrioventricular block, atrial standstill, sick sinus syndrome, asystole) | Hypovolaemia/dehydration | |
| Tachycardia (supraventricular tachycardia, ventricular tachycardia) | Situational (coughing, swallowing, straining during defecation or micturition) | |
A constant and adequate cerebral blood flow is required by the brain for its function, and any significant reduction or interruption of blood flow, even for a few seconds, can lead to falling and fainting. Cerebral blood flow is maintained by various mechanisms affecting arterial blood pressure (BP), which is the result of cardiac output (CO) multiplied by the total peripheral resistance (TPR; BP = CO × TPR). In turn, CO is determined by the volume of blood pumped by the heart (stroke volume; SV) per unit of time (heart rate; HR). This can be summarised as CO = SV × HR.
Therefore, changes in either SV, HR or TPR will affect arterial blood pressure and, ultimately, cerebral perfusion (Figure 1).
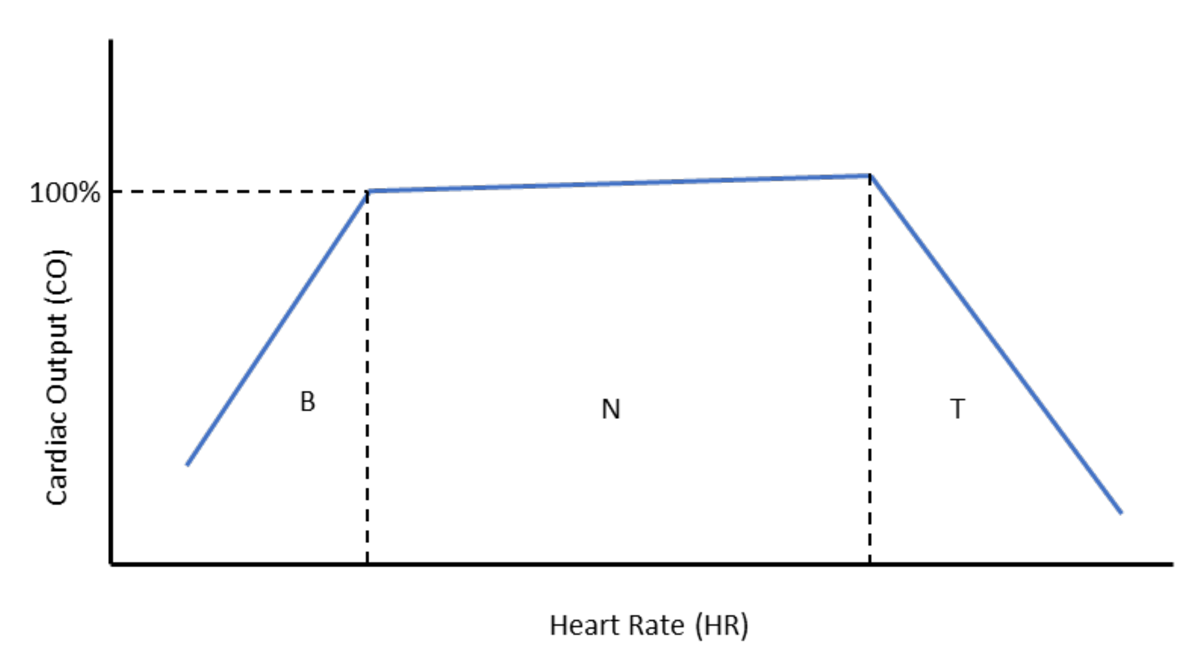
The pathophysiological mechanism of fainting secondary to outflow obstruction is rather intuitive, since any clinical condition that can significantly reduce SV will cause a drop in CO.
Congenital abnormalities, such as pulmonic and aortic or subaortic stenosis, can potentially cause severe right and left outflow obstruction, respectively, often accompanied by exercise intolerance and fainting events.
However, the most common form of outflow obstruction in cats is represented by dynamic left ventricular outflow tract obstruction secondary to systolic anterior motion (SAM) of the mitral valve, which is frequently (but not exclusively) observed in cases of hypertrophic cardiomyopathy (HCM).
However, fainting secondary to SAM is seldom observed in cats – unlike in human patients, where fainting episodes are commonly observed in cases of severe SAM, with or without concomitant left ventricular hypertrophy.
Physiological increases of HR, such as during exercise, have little effect on CO because when HR increases, SV decreases accordingly since the diastolic time is shortened, with subsequent reduced time for ventricular filling and reduced volume of blood ejected by the heart with the following contraction (SV).
Conversely, a physiological reduction in HR, such as during resting and sleeping, is accompanied by an increased diastolic time and associated ventricular filling, causing increased SV. However, when changes in HR become pathological (that is, tachycardia and bradycardia), CO drops significantly in both conditions.
Indeed, rapid and sustained tachycardia can critically reduce diastolic time to the point that SV becomes severely affected; vice versa, a profound bradycardia, despite the increased ventricular filling, can lead to a reduced CO (Figure 1).
Examples of syncopal events induced by rapid tachycardia and profound bradycardia are reported in Figures 2 and 3, respectively.
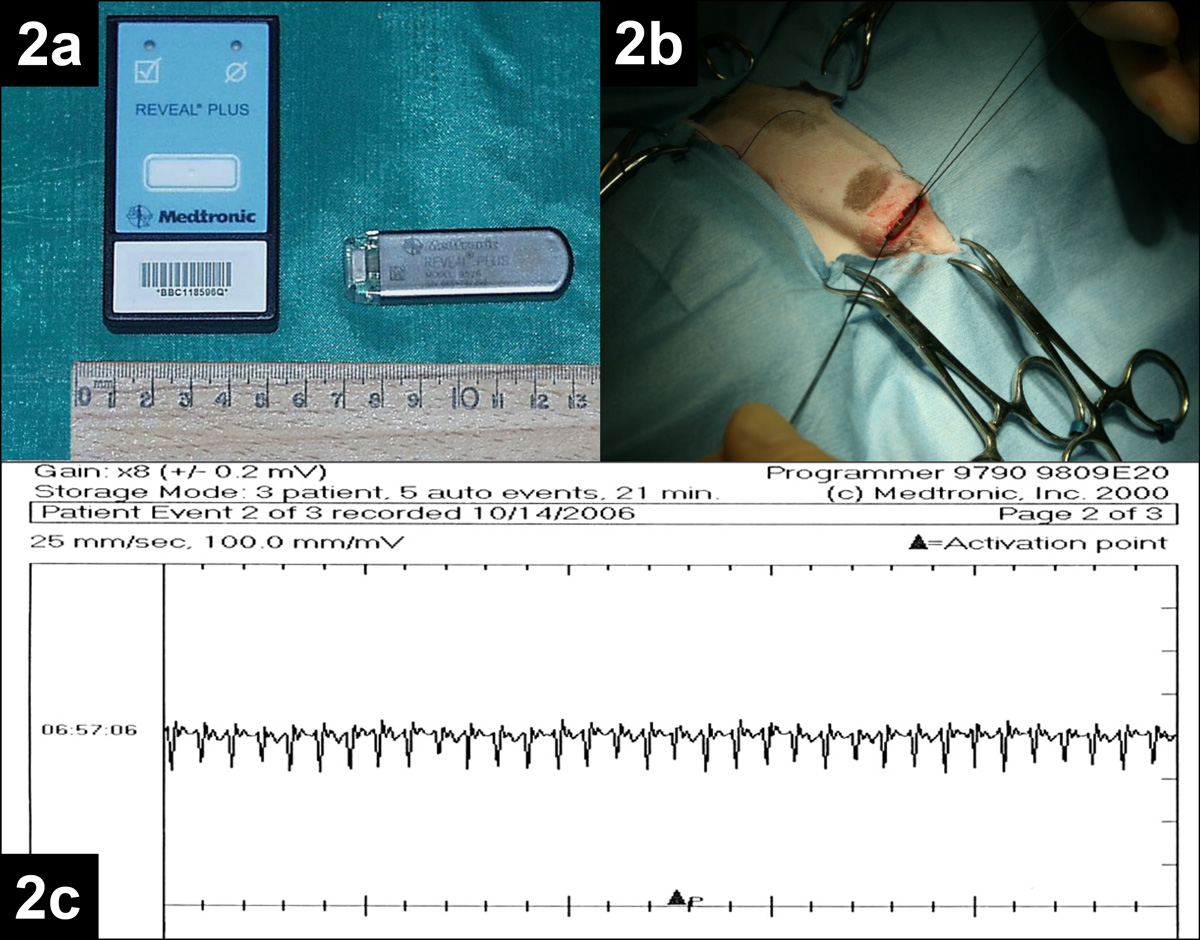
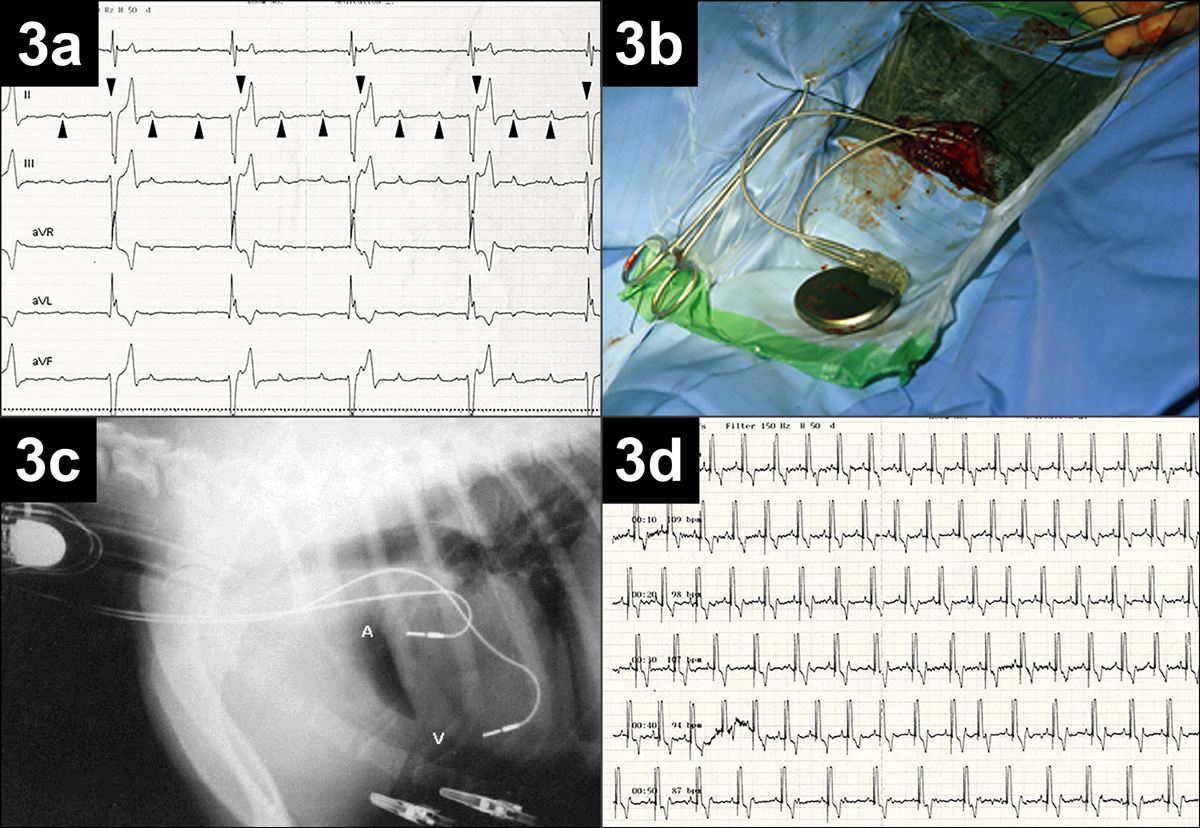
Myocardial disease, such as HCM, dilated cardiomyopathy (DCM) and arrhythmogenic right ventricular cardiomyopathy (ARVC) can occasionally cause fainting.
Although cardiac arrhythmias often accompany myocardial disease in dogs and cats, myocardial failure should also be taken into consideration. Significant myocardial dysfunction can severely affect CO, which can result in diminished blood flow in intramural coronary vessels and the rest of the body.
Similarly, all end-stage forms of cardiac disease will eventually be accompanied by myocardial dysfunction, reduced cardiac contractility and, ultimately, a decline in CO.
Furthermore, transient myocardial insults, such as myocarditis, can sometimes lead to falling and fainting events, usually secondary to arrhythmias or conduction disturbances.
Neurally mediated syncope is the most common type in people, comprising approximately 45% of cases, but it is also seen in dogs and, less commonly, in cats. It can be vasovagal, situational, or secondary to carotid sinus hypersensitivity. The pathophysiology of neurally mediated syncope is rather complex and originates from an increased sympathetic tone, which can sensitise and facilitate the activation of cardiac mechanoreceptors that occurs with very vigorous ventricular contraction, or by activation of C-fibres secondary to myocardial ischaemia and reperfusion.
The activation of these receptors triggers an abrupt, centrally mediated reduction in sympathetic activity, with concomitant parasympathetic activation, which results in vasodilation and bradycardia, and ultimately, a rapid profound decline of blood pressure.
An example of vasovagal syncope in a dog is reported in Figure 4.
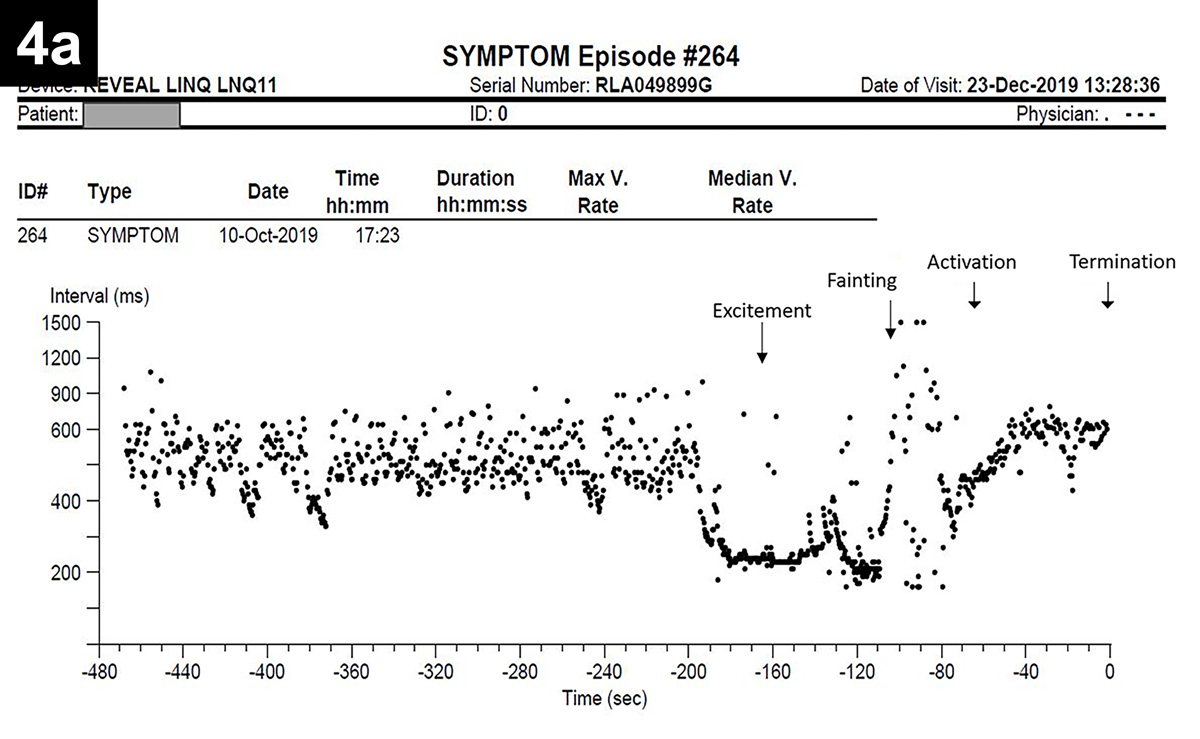 |
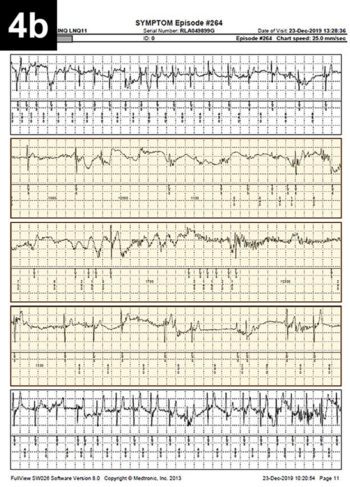 Figure 4. Oscar, a five-year-old male neutered pug, experienced several episodes of falling/fainting since he was a puppy, occurring on approximately a monthly basis. An implantable loop recorder (ILR) was used to determine the cause of Oscar’s fainting. Figure 4. Oscar, a five-year-old male neutered pug, experienced several episodes of falling/fainting since he was a puppy, occurring on approximately a monthly basis. An implantable loop recorder (ILR) was used to determine the cause of Oscar’s fainting. A few weeks after the ILR implantation, the dog was particularly excited, jumped on his owner’s lap and “keeled over” unconscious, with rigid legs and extended neck for approximately 30 seconds, before regaining full consciousness and gait. The owner used the dedicated ILR remote control (also named “Patient Assistant”) to record the event. ILR interrogation revealed the presence of a tachycardia-induced prolonged sinus arrest. The heart rate before the event was approaching 300bpm (period of excitement) and was abruptly interrupted by profound bradycardia lasting approximately 20 seconds during which the Patient Assistant was activated. Figure 4a shows the RR interval (inversely proportional to heart rate) that drops significantly during the period of tachycardia and rises suddenly during the fainting episode. Figure 4b shows the electrocardiogram trace recorded by the ILR during the period of profound bradycardia (highlighted in yellow). |
Situational syncope can be observed in dogs and cats following defecation or coughing. The mechanism of defecation syncope is likely associated with the increased intrathoracic and intra-abdominal pressure during defecation, which tend to reduce venous return and, subsequently, SV and CO. Profound bradycardia and hypotension are also observed due to a profound vasovagal stimulation.
These patients tend to have a history of straining prior to fainting, and this can cause activation of receptors in the intestinal wall, resulting in sudden hypotension and bradycardia. Situational syncope secondary to coughing has a mechanism very similar to the one explained previously. An example of cough-induced fainting is reported in Figure 5.
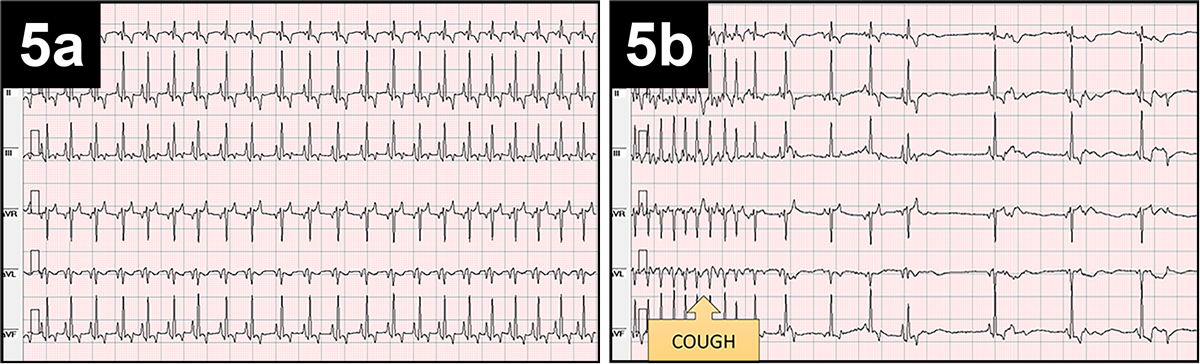
The transitory nature and the spontaneous resolution of fainting episodes represent a real diagnostic challenge.
In people, when no abnormalities are reported on the clinical history or physical examination, diagnosis may not be achieved in approximately 50% of cases, and similar figures are reported in small animal practice. Therefore, a good knowledge of efficacy and utility of various diagnostic tests is necessary to institute appropriate management promptly and efficiently.
In fact, a wrong choice of sequential tests may result in poor diagnostic yield and unnecessary costs.
Furthermore, a delayed diagnosis may occasionally result in sudden death. The presence of loss of consciousness is usually determined through a thorough history taking and its occurrence can be established using three historical features.
First, a loss of normal motor control is evident as flaccidity or stiffness, either of which can be accompanied by jerking movements, and postural control is lost, so that animals fall if they are in a quadrupedal or roll over if they are in sternal recumbency.
Second, normal responsiveness is lost (that is, the pet does not respond to the owner). However, it is important to note that, very often, their eyes can remain open even during a period of loss of consciousness.
Third, in people, patients experience amnesia for the event, and the authors believe this may also happen in animals.
Therefore, collecting the patient’s history in a thorough and consistent manner is fundamental, and appropriate questions should be formulated to understand whether the patient is experiencing loss of consciousness. Unfortunately, quite often, pet owners only report a vague description of “collapsing” events, while the clinician should determine if the reported episodes are accompanied by transient loss of consciousness.
Fainting episodes should also be characterised by rapid onset, short duration (usually no longer than 20 seconds) and followed by a spontaneous and complete recovery, although transient disorientation may be observed for several minutes in some cases.
Information obtained from pet owners should include history of cardiac disease or medications that may induce arrhythmias or hypotension; number and frequency of the episodes (in full detail); and identification of precipitating or trigger factors, including a detailed description of physical activity, time of the day, environmental conditions and degree of excitement prior to or at the time of the fainting episode. It is important to accurately quantify type and duration of the event, and obtain information (when available) about colour of mucous membranes and heartbeat during the events. Disorientation and behavioural changes following events, absence of pale or cyanotic mucous membranes, frothing at the mouth, lethargy after the event, and unconsciousness lasting for more than a few minutes would generally suggest seizure or a neurological aetiology, rather than fainting.
However, some patients – especially geriatric dogs – may experience prolonged syncopal events with involuntary defecation and/or urination, and may also display tonic-clonic activity and opisthotonos, mimicking seizures. For this reason, many pets experiencing fainting can be misdiagnosed with epilepsy and undergo expensive and often unnecessary diagnostic investigations, sometimes involving general anaesthesia.
Therefore, it is important to understand what happens before, during and after a falling event to distinguish, as much as possible, these two similar presentations.
In such cases, a detailed diary of events and video recording of falling episodes, even obtained with a simple mobile phone camera, can be extremely helpful to aid reaching of a diagnosis.
A full physical examination, with a particular emphasis on the assessment of the neurological and cardiovascular systems, is probably the most important component of the evaluation of a fainting patient.
Particular attention should be paid at the evaluation of the cardiovascular system (including blood pressure measurement), level of hydration and presence of concomitant neurological or musculoskeletal abnormalities. The presence of cardiac arrhythmias, heart murmurs and gallop sounds would be highly suggestive of an underlying heart abnormality, and should justify a complete cardiac evaluation.
Basic haematology and serum biochemistry have a low diagnostic yield, although they may occasionally detect severe anaemia, dehydration, electrolyte imbalances and musculoskeletal lesions, and so forth.
Measurement of the biomarker cardiac troponin I may reveal elevations consistent with an underlying myocardial insult, including myocardial ischaemia and myocarditis.
Echocardiography is a diagnostic test that should be considered in case of abnormal findings during the physical examination (such as heart murmur, arrhythmia, gallop sound and so forth), or in case of familial history/predisposition of cardiac disease.
However, the majority of fainting dogs and cats have unremarkable echocardiographic findings.
A resting electrocardiography diagnostic test rarely identifies the reason of fainting in dogs and cats. Indeed, fainting associated with an ECG abnormality is often missed due to the sporadic nature of fainting, even on a five-minute continuous ECG recording. Therefore, prolonged ambulatory ECG monitoring (Holter) can represent a more successful diagnostic tool. Nevertheless, even 24/48/72-hour ECG recording has a relatively low diagnostic value – especially when the falling episodes occur occasionally and can be missed during the recording.
The only exception for justifying Holter monitoring is for fainting episodes occurring several times a week. Alternatively, 7/14 day monitoring, only available via a limited number of Holter or ambulatory event recorders, provides a higher chance to achieve a conclusive diagnosis.
The diagnostic yield increases dramatically if an implantable loop recorders (ILR) is used in fainting animals, and these devices have an excellent utility in patients exhibiting infrequent or sporadic fainting events.
ILR reliability and accuracy has been reported in the veterinary literature with excellent results. These studies show that in dogs and cats with unexplained syncopal episodes, the manually activated ILR can provide invaluable diagnostic and prognostic information in almost all patients, by either confirming or disproving the association between fainting and arrhythmias or conduction abnormalities.
If a fainting event is observed during ECG recording (resting ECG, Holter monitoring or ILR), and the results are not suggestive of a cardiac aetiology, then other investigations should be considered, including abdominal ultrasound, to rule out mass lesions or internal bleeding, thoracic CT scan, MRI, electromyography, electroencephalography, cerebrospinal fluid collection and so forth.
The management of animals that experience fainting requires the recognition of the features of each of the major causes and, often, a good cooperation between different clinical specialities.
Treatment of fainting patients has three major goals: avoiding sudden death and prolonging survival, avoiding traumatic injuries sustained after falling and preventing further syncopal events.
The therapeutic approach mainly depends on the underlying cause. If a definitive diagnosis has not been achieved yet, pet owners should be instructed to keep their animal indoors to avoid potential trauma that may occur if the pet is left unsupervised. Conversely, if a diagnosis has been achieved, therapy should be aimed at controlling the underlying primary condition responsible for the transient loss of consciousness.
Treatment of sustained or paroxysmal rapid ventricular and supraventricular tachycardia, for example, can be attempted with antiarrhythmic medications. However, antiarrhythmic medications can decrease the pet’s HR and affect SV and cardiac output, potentially precipitating congestive heart failure in patients affected by a systolic dysfunction. Therefore, it is important to consider Holter monitoring a few days after starting anti-arrhythmic therapy, to monitor the appropriate response to treatment and possible residual presence of paroxysmal tachycardias.
The appropriate treatment of pets fainting as a consequence of significant bradycardia would be a permanent pacemaker implant in most situations. This type of intervention is available in most veterinary referral centres.
The exception to this is bradycardia secondary to electrolyte imbalances (such as hyperkalaemia in Addisonian patients or secondary to urethral obstruction), in which case therapy should be aimed at correcting the imbalance and managing the underlying condition.
Fixed outflow obstructions, such as aortic and pulmonic stenosis, can often be palliated with balloon valvuloplasty, which is a minimally invasive cardiac intervention using a catheter with a balloon on its tip that can be inflated at the level of the stenotic valve to stretch and open the partially fused cusps.
Therapy for neurally mediated syncope is seldom necessary. A careful history collection, with particular attention focused on identifying precipitating factors, should reveal the triggering events and avoidance of these circumstances (such as stress or excitement) is usually sufficient to drastically reduce the frequency and severity of fainting episodes.
Situational syncope can be successfully controlled by addressing and eliminating, when possible, the underlying condition (such as coughing or straining during defecation).
Pacemaker implant represents a controversial solution for neurally mediated syncope, but this solution might be considered if the fainting event is caused by very long cardiac pauses. The major limitation of pacemakers in treating neurally mediated syncope is their inability to control the systemic hypotension associated with the sudden vagally induced vasodilation.
When considering pacemaker implantation in dogs and cats with neurally mediated syncope, pacemakers should be programmed with specific pacing algorithms, such as HR hysteresis, which is a programmable feature that allows the pacemaker to start ventricular pacing only if the spontaneous rate falls below a critical HR and pacing continues at a programmed higher HR, unless intrinsic ventricular activity is sensed.
Therefore, when an intrinsic event is sensed, the higher rate of artificial pacing may compensate for the hypotension caused by the sudden reduction in total peripheral resistance.