14 Jul 2021
Carolina Fernández Sánchez discusses a case involving an eight-year-old male neutered Staffordshire bull terrier in the latest in the Vet Times Case Notes series.

An eight-year-old male, neutered Staffordshire bull terrier was referred for episodes of sporadic collapse, bradycardia and polyuria-polydipsia (PU-PD).
The syncope was not related to exercise stimulation and the owner described the collapse like “falling asleep”. He also presented with vomiting and lethargy. He never travelled outside the UK and was fully vaccinated.
On presentation the dog was bright, alert and responsive. Mucous membranes were pink, with capillary refill time less than two seconds. Bradycardia was detected (40bpm) with sinus rhythm and good-quality femoral pulse without deficits.
Mean arterial pressure was normal (130mmHg). Auscultation of the lungs and heart were unremarkable. The remainder of the physical examination, including orthopaedic and neurological system, was unremarkable.
The differential diagnoses for bradycardia are:
Blood samples were taken for routine haematology, biochemistry and urinalysis to rule out a metabolic problem. Haematology was unremarkable.
Biochemistry revealed mild hyperglycaemia (15.5mmol/L) and mild hypercholesterolaemia (7.1mmol/s). Fructosamine was mildly elevated (541umol/L). Bile acid stimulation test was unremarkable, with values of 8μmol/L fasting bile acid and 15μmol/L postprandial bile acids.
The patient was hospitalised for a blood glucose curve to detect a possible iatrogenic hypoglycaemia. The glucose was measured hourly and the nadir of blood glucose was 4.6mmol/L, which showed a good control of glycaemia – so hypoglycaemia was unlikely to be the cause of the collapse.
His water intake was 200ml/kg/day. PU-PD in a dog has been defined as water consumption greater than 100ml/kg/day.
Urine analysis revealed urine specific gravity (USG) 1.002 with no glycosuria, unremarkable sediment and negative culture. The USG was checked a few times on different days and was less than 1.010 in all cases. This is extremely low for a normal dog.
A pre-adrenocorticotropic and post-adrenocorticotropic hormone stimulation test, and a low dose dexamethasone suppression test were also performed to rule out hypoadrenocorticism and an HAC, respectively. The results of these tests were normal.
An ECG was indicated to detect if any significant bradyarrhythmia existed.
Figure 1 shows the ECG. Heart rate is 50bpm. R-R distance is regularity regular. All the P waves are normal and look the same. P waves exist for every QRS complex, but a QRS complex does not exist for every P.
PR interval is progressively longer until QRS is missed and then recaptures occur. QRS complex duration is normal (0.04sec to 0.12sec). A sinus bradycardia with a second-degree atrioventricular (AV) block Mobitz type I exists. This type of AV block is likely to result from an elevated vagal tone.
A Holter monitor was placed for 24 hours to look for an intermittent arrhythmia. The heart rate varied between 40bpm when the dog was resting and 190bpm when he was excited.
The atropine response test was performed to distinguish if the bradyarrhythmia was secondary to a systemic disease (high vagal tone), or due to primary cardiac conduction disturbances or cardiac disease.
In this case, the atropine test confirmed a high vagal tone cause. It showed a good response with the AV block being eliminated and the sinus rhythm increased from between 40bpm and 50bpm to between 120bpm and 150bpm. This also happened when stimulating the dog.
During the hospitalisation the dog had a collapse episode. He got excited when he was fed, became unsteady and fell asleep. During the collapse, he was conscious as his eyes were open and he was capable of following moving objects. It lasted a few seconds and he came back to normal with tactile stimuli.
The heart rate was around 40bpm during the episode with plasma glucose 17mmol/L; potassium was normal. This ruled out the electrolyte abnormality and the hypoglycaemia as the cause of collapse.
Thoracic radiographs were performed to investigate causes of hypoxaemia and cardiac disease, and abdominal ultrasound to help to rule out a hepatopathy, adrenal or renal disease that may cause collapse and PU-PD. They were unremarkable.
At this stage, metabolic and cardiac disease were considered unlikely to be the cause of collapse with the previous work-up. A high vagal tone seems to be the cause of the bradycardia and hence the collapse. Ophthalmic, respiratory and abdominal causes of high vagal tone have been excluded already. Conversely, neurological causes seem the most likely cause of bradycardia and collapse.
Neuromuscular disorders or peripheral neuropathy were ruled out as it does not cause symptoms such as abrupt onset of the collapse with a sudden recovery.
However, the type of collapse resembles a narcoleptic episode in which the dog usually remains conscious with the eyes open. After a few seconds the dog may fall into sleep, often with rapid eye movements, muscle twitching and/or slow repetitive movements of the forelimbs and hindlimbs.
The attacks can be interrupted by calling the dog’s name or patting its body, and the muscles are always flaccid (cataplexia) and never stiff (like in most seizure attacks). In contrast with some forms of epilepsy, salivation, urination or defecation are not observed during cataplectic attacks. Neither prodromal nor postictal periods are present.
On the other hand, a hepatic, renal and some metabolic diseases have been excluded as a cause of PU-PD. Psychogenic polydipsia and HAC seem less likely on the basis of other clinical signs and the previous test. Hence, DI turned out the most likely cause of hyposthenuria.
DI is a metabolic disease that could be central or nephrogenic. In the central DI (CDI) presentation a lack of arginine vasopressin (AVP) is produced by the hypothalamus and, hence, it is not released by the neurohypophysis. This hormone is responsible for water absorption in the renal tubules. In nephrogenic DI (NDI) the nephron is insensitive to the action of antidiuretic hormone. The clinical signs are hypernatraemia and PU-PD, and USG less than 1.010.
The desmopressin test was used for the confirmation of DI, and to distinguish between central and nephrogenic presentation. Desmopressin is an analogue of the hormone AVP and it does concentrate the urine in most of the cases of CDI, with no response or very little in NDI.
Two types of CDI exist. In complete CDI the osmolarity will increase by 50% or more than the initial USG value. In partial CDI the osmolarity would increase by 15% or greater.
The patient was hospitalised for a few days with desmopressin (one drop in the eye three times day) to assess the treatment. His water consumption on desmopressin decreased from 200ml/kg /day to around 80ml/kg/day and the USG increased up to 1.035. Therefore, the diagnosis is a complete CDI.
CDI could be produced by a pituitary-hypothalamic tumour or malformations, metastasis, inflammatory disease, head trauma, congenital abnormalities or idiopathy.
The owner was offered further imaging to assess for hypothalamic/pituitary abnormalities. An MRI of the brain was chosen in this case as, in contrast with a CT, it has greater range of available soft tissue contrast, depicts anatomy in greater detail, and is more sensitive and specific for abnormalities within the brain itself. The sequences T1w, T2w, flair and gradient echo were performed.
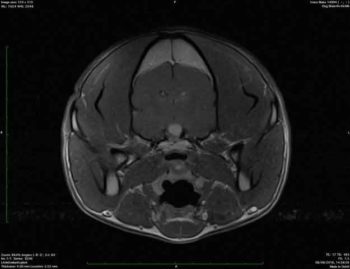
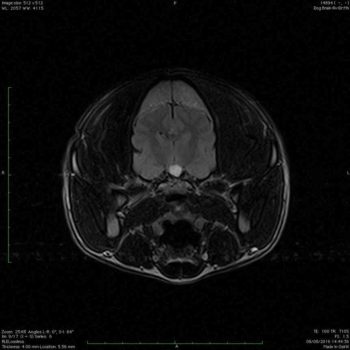
A well-defined capsule structure was located in the sella tunica, causing mild elevation of the hypothalamus and significant compression of the neurohypophysis. The structure was hyperintense in all the sequences (Figures 2 and 3). CSF cytology and protein were unremarkable.
The differential diagnosis would be pituitary cyst lesion, neoplasia (for example, craniopharyngioma) and less likely Rathke cyst or granuloma. Rathke cyst is usually incidental and asymptomatic, so it is not possible to be the clinical signs.
Due to the location of the mass, brain biopsy was unfeasible, so a definitive diagnosis was not possible in vivo, but it could potentially be the cause of the central DI. Moreover, due to the clinical signs narcolepsy is still suspected.
Dogs with narcolepsy have a defect in the neurotransmitter hypocretin, which is involved in maintaining the awake state and is produced by the ventrolateral nucleus of the hypothalamus.
Hypocretin has a role in the autonomic nervous system by stimulating the release of norepinephrine and serotonin. These hormones both inhibit the release of acetylcholine. When this inhibition doesn’t happen it leads to bradycardia, and an inhibition of the general somatic efferent lower motor neuron in the spinal cord and the brainstem that caused flaccid paralysis and consequent narcolepsy.
Narcolepsy could be inherited or acquired. Inherited narcolepsy seems unlikely in this case as it is due to a mutation in a gene and the clinical signs often occur as early as four weeks of age. However, acquired narcolepsy seems likely in this case.
It is usually seen in older dogs, suggesting a dysfunction of the neurons in the ventral nucleus of the hypothalamus. In this form, a depletion of hypocretin in the CSF has been recognised. The cause is unknown, but an autoimmune inflammation or drug-related has been proposed. Measuring the concentration of CSF for the confirmation of this form of narcolepsy could not be performed in this case.
DI is usually treated with desmopressin. It is available in preparations for parenteral, intranasal and oral administration.
The intranasal preparations given in the conjunctival sac was chosen in this case.
The dose is 1.5 micrograms to 4 micrograms (one drop) three times a day and it should be gradually adjusted as needed to control signs of PU-PD.
The treatment for narcolepsy is recommended when the episodes become frequent and it interferes with the quality of life.
One of the drugs that could be used is imipramine, which is an antidepressant that inhibits the reuptake of norepinephrine and has anticholinergic actions. The dose recommended is 1mg/kg twice a day orally.
The dog responded very well to the treatment. PU-PD resolved as well as the narcolepsy. Rechecks were done regularly to check USG, glucosuria and blood glucose. Fourteen months after the diagnosis of CDI the dog was still alive. The fact that the dog is responding to the treatment of narcolepsy contributes to the diagnosis.
The author would like to thank Andrea Di Bella for his help in this clinical conundrum.