13 Aug 2024
Diseases such as maedi visna, ovine pulmonary adenocarcinoma and ovine Johne’s disease vary in UK prevalence, but all have huge economic impact and health implications. David Charles focuses on the big “iceberg” diseases in sheep, including screening and testing.

Image: Melissa Collins (expanded by AI)
Iceberg diseases are often slow to be detected or diagnosed, such that the percentage of a flock showing signs of clinical disease does not represent the total number of infected animals.
Therefore, we have the age-old adage that “clinical cases are just the tip of the iceberg”: for each clinically affected animal, many more will be infected or harbouring the disease that are not yet displaying clinical signs. As a result, production within the flock will be significantly reduced.
We now know iceberg diseases that cause chronic wasting can reduce cull ewe value by 20% to 30% (Ogden, Davies and Lovatt, 2019; Bell et al, 2021). The economic impact to our clients’ results from more than just dead ewes.
The five classic “iceberg diseases” affecting sheep in the UK are ovine pulmonary adenocarcinoma/jaagsietke (OPA), caseous lymphadenitis (CLA), maedi visna (MV), ovine Johne’s disease/ovine paratuberculosis (OJD); and border disease (BD).
While specific prevalence data is hard to come by, two recent publications’ data are summarised in Table 1.
| Table 1. Prevalence data for iceberg diseases within the UK flock | ||
|---|---|---|
| Disease | Estimated UK flock prevalence (Agriculture and Horticulture Development Board, 2019) | Percentage of flocks disease found in (study of cull ewes on 75 farms; Bell et al, 2021) |
| Border disease | 30.4% to 37.4% | 0% |
| Maedi visna | 2.8% | 3% |
| Ovine Johne’s disease | 64% | 32% |
| Caseous lymphadenitis | Up to 18% of flocks have at least one seropositive ram | 1% |
| Ovine pulmonary adenocarcinoma | 0.9% to 5.6% | 11% |
With clinical cases not being a representative way to assess the prevalence of disease, or risk of infection within a flock, regular screening or monitoring is essential for flock health.
In the past few years, we have seen several funded projects promoted to vets and sheep farmers, with a view to increase the understanding of “iceberg diseases” and their prevalence within the UK flock. Screening within flocks can also inform farmers about their risk of infection or outbreak, and allow prevention protocols (such as accreditation and buyer-selection methods) to be implemented.
One such project was the APHA “thin ewe” project, which ran from November 2020 to May 2021. The results of this can be seen in Table 1. Perhaps, most strikingly, this screening project demonstrated that one or more iceberg diseases were present in 41% of flocks.
For Scottish farmers, funding can be drawn down from the Scottish Government’s “preparing for sustainable farming” initiative, allowing “investigations” up to the value of £250 into the iceberg disease status of a flock. To fulfil the criteria, farmers need to work with vets to ensure both testing and a prevention or control plan are carried out (SRUC Veterinary Services, 2023).
Both initiatives are good examples of government funding to improve flock health and limit the impact of iceberg diseases. However, compliance and awareness of such projects remain limiting factors, and vets should work to stay informed of different funding sources available to their clients, so that as many farms as possible are engaged with and can benefit.
Regular monitoring is easily done – especially as veterinary engagement with sheep farmers grows through projects such as flock health clubs, animal health and welfare pathway visits, and an increased understanding of the impact of iceberg diseases (Gascoigne et al, 2019; Bell et al, 2021; Defra, 2022).
An example approach to effective screening for iceberg diseases in a flock is laid out in Table 2; however, cost and compliance will affect how a screening programme is rolled out.
| Table 2. One approach to routine screening for iceberg diseases within a flock | |
|---|---|
| Disease | Screening approach |
| Ovine pulmonary adenocarcinoma | 1. Postmortem examination of a proportion of cull ewes and fallen stock. Use of histology to confirm diagnosis if lesions are observed 2. Transthoracic ultrasonography of cull ewes to identify candidates for postmortem examination. Operator experience is a limiting factor in the accuracy of the test (Davies et al, 2022) |
| Caseous lymphadenitis | 1. Serology antibody ELISA testing in a group of mature breeding ewes, and testing of all rams |
| Maedi visna (MV) | 1. Serology antibody ELISA testing in all rams, and a cohort of ewes (if unaccredited) 2. Serology antibody testing of the proportions and fre- quency outlined by the SRUC MV accreditation regulations (SRUC, 2022a) |
| Ovine Johne’s disease (OJD) | 1. Postmortem examination of thin ewes sent as culls, with or without histopathology 2. Faecal PCR testing of pooled samples provides 80% to 85% sensitivity (Begg and Whittington, 2020) and is an affordable, useful test to determine flock prevalence 3. Pooled faecal culture, with 92 per cent sensitivity (Windsor, 2014), helps to identify OJD within a flock and clarify risk of shedding and likelihood of environmental contamination. However, results take 8 to 12 weeks (Moredun Research Institute, 2021; SRUC, 2022b) |
| Border disease | 1. Serology antibody ELISA testing in homebred new season lambs for exposure or infection 2. Antigen PCR test of purchased rams (during quarantine phase or before arrival) to identify PI status |
Effective screening and monitoring also relies on strict biosecurity and quarantine. Testing new arrivals – especially rams – is an essential part of keeping iceberg diseases out of a flock. The author has worked with multiple flocks that have suffered significant losses, and outbreaks of iceberg diseases, because rams and ewes have been purchased of unknown status.
Caused by the jaagsietke sheep retrovirus (JSRV), OPA-affected sheep develop bronchioloalveolar adenocarcinomas, which account for 70% of all tumours recorded in sheep (Bell, 2008). JSRV has a long incubation period, with clinical signs rarely observed before affected animals are two to four years old. Some breed predisposition is observed, with Scottish blackface sheep – and their crosses – being significantly over-represented (SAC Veterinary Consulting Services, 2013).
Affected animals shed large amounts of virus, and transmission has been demonstrated via oral secretions, milk and colostrum. Environmental survival reports are variable, with the Icelandic elimination programme only resting pasture for two months between groups.
Clinical signs include a loss of body condition score (BCS) and exercise intolerance when gathered. As the disease progresses, tachypnoea (exacerbated at times of stress or in hot weather), abdominal breathing, open mouth breathing and nasal discharge are frequently observed.
Diagnostic tests are outlined in Table 3; however, vets should be aware that the wheelbarrow test is not 100% reliable, with approximately one out of three positive ewes producing no significant fluid from the nasal passageways; therefore, providing a negative test result. Vets should only carry out the test if they have the means of immediate euthanasia to hand, as it causes extreme distress to affected ewes.
| Table 3. Diagnostic testing for iceberg diseases in a suspect animal | |
|---|---|
| Iceberg disease | Diagnostic test |
| Ovine pulmonary adenocarcinoma | 1. Wheelbarrow test 2. Transthoracic ultrasonography 3. Postmortem examination |
| Caseous lymphadenitis | 1. Serology antibody ELISA 2. Western blot for definitive diagnosis 3. Postmortem examination (high zoonotic risk and risk of infection spread, so extreme care is recommended if done on farm or in practice) |
| Maedi visna | 1. Serology antibody ELISA 2. Agar gel immunodiffusion test |
| Ovine Johne’s disease | 1. Serology antibody ELISA (high specificity of 98% to 100% [Begg and Whittington, 2020] is useful for diagnosis, but low sensitivity means it is less useful for screening) 2. Faecal PCR 3. Postmortem examination plus histopathology |
| Border disease | 1. Serology AB ELISA (paired testing three weeks apart) 2. PCR for antigen (blood or spleen or milk) – aborted fetus, live lamb/ewe, dead lamb |
Postmortem examination remains the most reliable method for diagnosing OPA, and examination of thin cull ewes should be carried out as part of a screening process in flocks. On postmortem, affected ewes have characteristic large tumours that are solid and grey; they may contain abscesses or areas of necrosis. Secondary bacterial infections are common, with frothy fluid in the airways. Histology provides a definitive diagnosis.
Transthoracic ultrasonography (TTUS) is used by some practitioners (Tables 2 and 3) to detect OPA lesions. Ultrasound of OPA-positive ewes should reveal extensive hypoechoic regions in the ventral areas of the lungs, in sharp contrast with the normal lung tissue. The theory is that early detection via TTUS provides economic and welfare benefits, while also reducing the shed of JSRV and the risk of it spreading within the flock. The approach is well described by Scott and Cousens (2018).
Using TTUS to detect OPA lesions is operator dependent, and significant numbers are required for operators to gain sufficient experience. However, Davies et al (2022) questioned if TTUS alone is sufficient, as they demonstrated that even with an experienced operator, an overall accuracy of only 24% was achieved and that gross postmortem examinations (which can be performed on farm) are still required. What was more promising was the fact all ewes flagged by the operator had lung pathology at postmortem, even if 76% were not due to OPA.
Management of OPA is difficult due to the chronic nature of the disease, large amounts of virus shed by infected animals and the difficulties of detection. Regular inspection of adult ewes, culling of affected animals and removal of lambs from infected ewes (possibly even snatch lambing) will be beneficial.
Keeping ewes in single-age groups to prevent mixing of old and young has been proven to be beneficial. Where possible, minimising the gathering of ewes in close-contact on affected farms can reduce the spread. As with the other “iceberg diseases”, effective quarantine and biosecurity measures are important to prevent introducing the diseases.
Caused by the gram-positive bacterium Corynebacterium paratuberculosis, CLA is an incurable bacterial disease primarily affecting small ruminants. It is zoonotic, and reports of infection in humans dealing with sheep are reported.
CLA affects the lymphatic system, with clinical signs including abscesses in lymph nodes (abscesses in the parotid or submandibular lymph nodes are frequently the catalyst for veterinary involvement), weight loss, wool loss and death. However, many animals do not show specific clinical signs, as the visceral form of the disease cannot be detected on clinical examination.
Infection occurs directly through aerosol spread between ewes in close contact, or indirectly through contaminated shearing equipment.
Diagnostic testing is described in Table 3. Lancing of abscesses is not recommended, as complete bacteriological cure cannot be guaranteed (even after antimicrobial treatment due to the intracellular nature of the bacteria; Davis, 1990) and it will risk environmental contamination.
Infected animals should be culled, and snatch-lambing may be required. Running a closed flock is the best approach to keep disease from a flock. All bought-in sheep (including rams) should be quarantined for eight weeks, and undergo a thorough clinical examination at the time of purchase and before mixing with the rest of the flock.
Serology AB ELISA is also recommended before purchase or mixing. No vaccines are licensed in the UK, and serological testing cannot differentiate between vaccinated and infected animals (Gascoigne et al, 2020).
C paratuberculosis can persist in the environment for more than eight months, and effective disinfection of sheds, equipment and handling facilities should be undertaken if the disease is detected in a flock.
Caused by Mycobacterium avium subspecies paratuberculosis (MAP), OJD is underdiagnosed in the UK, and flocks with a high prevalence record mortalities due to OJD of 5% to 15%. As with Johne’s disease in cattle, young animals are more susceptible to infection by MAP, with clinical disease presenting later in life after a prolonged incubation of three to five years (Moredun Research Institute, 2021; Worsley and Davies, 2022). Sheep are susceptible to both type S (sheep) and type C (cattle) strains of MAP.
Clinical signs are those of “ill thrift”, and OJD should be on all vets’ differential diagnosis list for adult ewes with “ill thrift”. Chronic loss of BCS with or without diarrhoea (unlike cattle), hypoalbuminaemia, hypocalcaemia and occasionally oedema are all detected.
On PME, changes to the terminal ileum, jejunum and colon are frequently observed. Typically, this presents as slight thickening and darkening/reddening of the mucosal lining with or without corrugation.
MAP is highly resilient, surviving in the soil for 47 months, water for 9 months and slurry for 11 months. Transmission occurs in several ways: faecal-oral, in colostrum/milk, in utero and via semen.
Diagnosis is discussed in Table 3.
Disease control is best achieved through aggressive culling of animals with poor BCS, targeted postmortem examination and sourcing negative replacements where possible. If economically viable, testing the entire flock (serum ELISA) has some benefit, otherwise targeted sampling of at least thin ewes at weaning (that are not excluded due to dentition, udder changes or other causes) with a pooled PCR test. Replacement rams should be tested before purchase (ELISA).
Vaccination is possible and may be beneficial in flocks with a high prevalence to reduce shed and environmental contamination. However, serology cannot determine between vaccinated and infected ewes, and this must be factored into any monitoring and prevention strategies.
Caused by a lentivirus, MV has two forms: maedi presenting with dyspnoea, heavy lungs and respiratory problems, and visna presenting as a progressive neurological disease. Disease prevalence is continually increasing. In 2012, some 100,000 ewes were believed to be infected and this number will have grown exponentially (Ogden et al, 2019).
Current estimates of prevalence are in Table 1. Regional variation also occurs, with 14% to 15% of flocks in Leicestershire and Gloucestershire infected.
Spread is through close contact via the aerosol route, or in the milk or colostrum of infected animals. Transfer through placenta or semen is also possible; however, these do not lead to the majority of infections.
The disease has a long incubation period, with clinical disease rarely observed in animals aged less than three years old. Clinical signs of maedi are that of a progressive interstitial pneumonia. Loss of BCS, dyspnoea, and fatalities are observed in clinical cases (Figures 1 and 2). Infected animals will produce antibodies; however, they remain lifelong carriers (Ogden et al, 2019).


Figures 1 and 2. A ewe with clinical maedi visna disease. The loss of body score condition is evident in the animal. Images: Melissa Collins
On postmortem examination, enlarged, heavy lungs are observed with imprints from the ribs. Enlarged mediastinal lymph nodes are also seen. Secondary bacterial pneumonia (often Mannheimia haemolytica) is common.
The ewe in Figures 1 and 2 exhibited clinical signs of disease. However, several other ewes in the same flock were MV-positive, without such pronounced clinical signs – demonstrating the iceberg concept of this disease.
Diagnosis is discussed in Table 3, where it is noted that postmortem examination and agar gel immunodiffusion testing are not always required. Many vets and farmers will advise culling if positive ELISA results are reported. At a flock screening level, multiple tests are required, as the time to seroconversion can vary from weeks to months, and animals with a low level of antibody can become transiently seronegative.
ELISA has been reported to have 99.4% sensitivity and 99.3% specificity. Milk ELISA is also available and may be more practical in milking flocks or meat flocks after lambing. With a long incubation period, control measures and programmes may take several years (short of entirely destocking and repopulating from accredited negative flocks).
Many non-accredited flocks opt for eradication by repeated testing and culling. Ewes older than 12 months of age are tested twice a year, with positive ewes culled alongside any offspring less than 12 months of age. With this method, eradication can be achieved in one to three years, if replacements are sought appropriately.
Other methods exist, including running two flocks entirely separately (one seronegative, one seropositive), with repeated testing on the seronegative flock and removal of any subsequent seroconverts.
Replacements are only kept from the seronegative flock: over time, the size of the seropositive flock reduces to zero, leaving a negative flock. An accreditation scheme exists, and vets are encouraged to be familiar with it, its rules and how to sign flocks up (Scotland’s Rural College, 2022a).
Caused by a pestivirus, BD can present in a flock in several ways.
Clinical signs vary depending on when the ewe is infected (Figure 3). Fetal resorption is a feature of early infection of ewes by BD, and the disease should be remembered if more empty ewes than usual are observed at scanning.
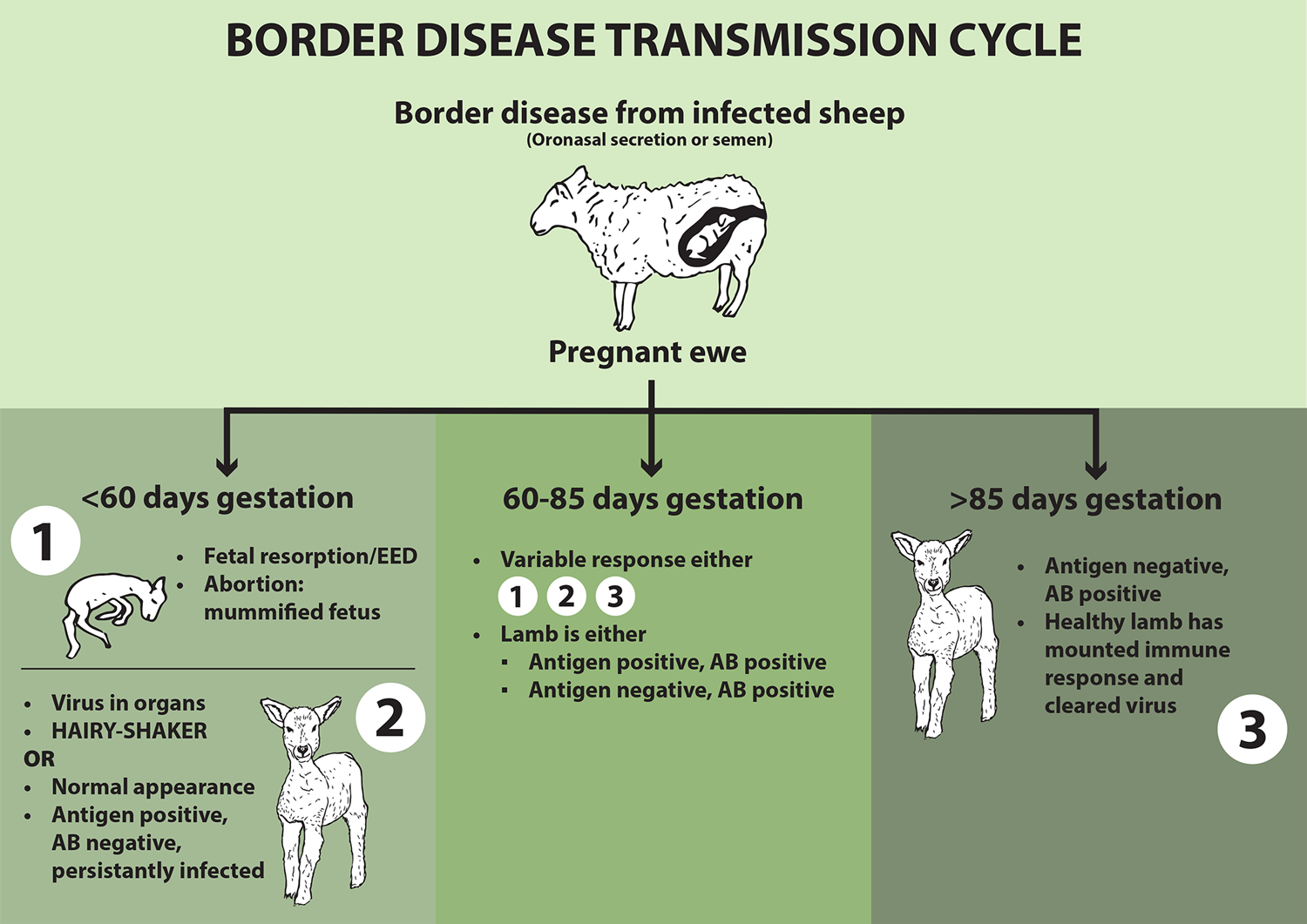
Figure 3. The impact of border disease, based on when the ewe is infected by bovine viral diarrhoea virus.
Not all persistently infected (PI) lambs present as hairy shakers (domed head, weak, long coat, tremors and increased mortality), and the ones that do not present as such are at greater risk of going unnoticed and spreading disease later in life.
Spread occurs through oro-nasal infection, in-utero spread or via the semen of infected rams. Introducing a transiently infected or PI animal into a naive flock can have significant and long-lasting impact.
Diagnostic testing is discussed in Table 3. It is important to remember to take paired samples for serology AB ELISA, allowing time for seroconversion.
Control centres around culling all lambs from groups with a high incidence to avoid the risk of retaining PI animals. Testing of rams before introduction to the flock for PI status is just as important. The author knows of no studies or data investigating the benefit or safety of BVD vaccines for cattle in UK sheep.
Iceberg diseases remain an important cause of losses in the UK flock, with economic losses arising from more than just dead animals. Having appropriate control and prevention methods in place for these diseases, including appropriate screening measures, is essential to the health and productivity of any flock.