7 Aug 2017
Hany Elsheikha considers the most economically important endoparasitic worm species in cattle and management options.

With the world’s population projected to increase to more than nine billion, the need to meet people’s nutritional demands for animal products will be greater than ever. Ensuring sustainable agricultural and livestock production systems will be essential to addressing this global challenge to food security. However, despite advances in controlling worms in cattle – especially in recent years – worm infections remain an important challenge to a sustainable agricultural economy.
The adverse impact of worm infections on the health and productivity of affected cattle, and the associated economic losses, indicate these infections might affect the ability to meet the future global demand for animal products; thus, new, innovative therapeutic and preventive ways are required if this demand is to be met. In this article, data on the clinical manifestations, diagnosis and control of worm infections in cattle are reviewed and discussed.
By 2050, the world’s population is projected to grow by one-third – reaching between 9 and 10 billion. With this anticipated rise, a substantial increase in per capita meat and dairy consumption is also expected.
The increasing demand for calories from animal products will probably double, highlighting the importance of developing and maintaining a sustainable animal agriculture system. However, a rapidly growing human population, combined with a warming climate and changes in the epidemiology of cattle parasites, represent major challenges for efficient and sustainable agricultural and livestock production systems. For example, endoparasites are responsible for significant economic losses in cattle because they can compromise health status, growth rate, feed consumption, welfare and productivity of affected animals.
In the meantime, cattle farming is of a major socioeconomic importance in developed and developing economies. To this end, the need for building a sustainable agricultural system that addresses this global food security challenge and meets the nutritional demands of the increasing human population for animal products is compelling. In this article, the author provides a summary of the endoparasitic worms infecting cattle and their clinical impact, diagnosis and management.
The endoparasites of cattle include a large group of worms/helminths – such as trematodes (flukes), cestodes (tapeworms) and nematodes (roundworms) – and protozoal species (for example, Cryptosporidium parvum, Eimeria species, Neospora caninum and Trichomonas species). Due to space limitations, this article focuses on the most economically important endoparasitic worm species.
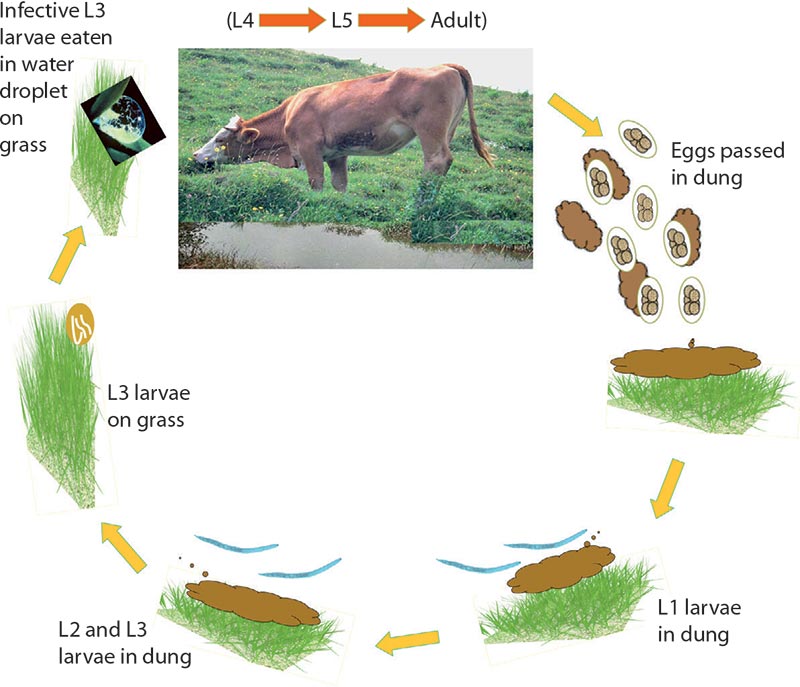
Cattle can be infected with a number of gastrointestinal (GI) roundworm species, including Haemonchus placei (Figure 1), and Ostertagia ostertagi in the abomasum, and Nematodirus species in the small intestine. Other genera, such as Trichostrongylus, Oesophagostomum and Trichuris, can also cause disease in cattle. Several species of the genus Cooperia are highly prevalent in cattle aged below two years. The life cycles of these parasites are similar during the environmental phase outside the host, but vary considerably inside the animal (Figure 2).
Interestingly, many nematodes undergo a condition called hypobiosis, or arrested development. Hypobiosis is a cessation of metabolic activity of the developing juvenile stages within the host whereby the parasite survives by evading unfavourable conditions in the host and/or environment. When the host is stressed by factors, such as parturition or lactation, the arrested juvenile parasites complete their development, mature and begin egg production.
GI nematode parasites cause a condition known as parasitic gastroenteritis, which is the most important profit-limiting group of parasitic diseases in the cattle industry. In general, most of these parasites seem to be important in the young, then, as resistance develops, become less important. One exception is O ostertagi, which can cause disease in adult cattle. Clinical signs vary based on the part of the GI tract affected, and the developmental and reproductive cycles of the parasite in the infected cattle.
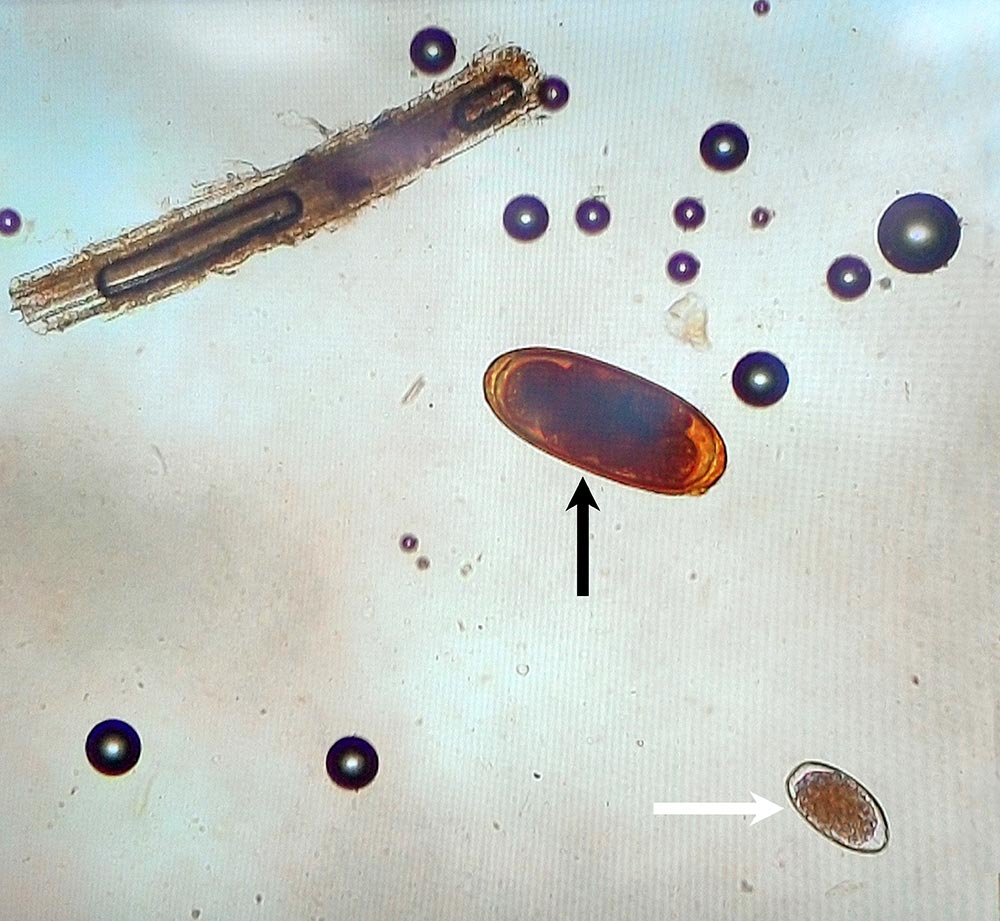
Traditionally, infections with nematode parasites have been diagnosed based on counting parasite eggs using a faecal flotation method (for example, faecal egg count; FEC). However, this method is labour-intensive and provides limited information on the infecting species because, apart from Nematodirus species (Figure 3), eggs of most GI nematodes are morphologically indistinguishable since considerable overlap exists between the size of eggs from different species. Some cost savings could be achieved by performing a composite FEC test using pooled faecal samples from a group of animals rather than FEC of individual samples.
Larval culture is another method used to identify which genera of nematodes are present in faecal samples. This requires the incubation of faecal samples for one to two weeks to allow eggs to mature and infective third-stage larvae (L3) to hatch, which are identified using published identification keys on size and morphology of the important nematode genera and species.
Although useful, this approach is laborious and potentially inaccurate due to the overlap in the morphometric characteristics of these genera. Larval culture also requires experienced operators to avoid errors in the identification of the parasite species. In addition, the temperature at which faeces are incubated can affect the abundance of the infective larvae harvested and counted, so the results of larval culture may not reflect the original population of parasite eggs that would eventually go on to pasture.
Nucleic acid amplification assays (for example, PCR or reverse transcription PCR) have been developed for the diagnosis of GI nematode infections in cattle. A robotic PCR platform for the genus and species-specific identification of GI nematodes from bovine faecal samples has been developed (Roeber et al, 2017). This PCR, which takes one to two days, was found to be more than 90 per cent sensitive and specific for the identification of the important GI nematodes in infected cattle, compared to traditional microscopic methods. This assay is simple to use and the operator requires no knowledge or experience to identify the nematodes present.
GI nematode infections can also be monitored based on serological methods, such as ELISA assays based on crude saline extract or whole worm O ostertagi antigen. Other biochemical parameters can also be considered in the diagnosis of GI infection in cattle.
Treatment includes avermectins, milbemycins, benzimidazoles, probenzimidazoles; levamisole and morantel. Not all compounds are suitable for controlling hypobiotic larvae. Control methods differ widely according to climatic conditions, alternate grazing management options and the presence of anthelmintic resistance (AR). The major aim is to maintain safe grazing by reducing pasture contamination. Integrated management schemes that reduce dependence on drug usage are preferred to prevent AR.

Paramphistomum species (trematodes of the family Paramphistomidae) have a worldwide distribution and are considered to be important parasites of a number of ruminant species, particularly in tropical and subtropical areas. However, concern is growing about the introduction, potential geographic expansion and associated economic consequences of rumen flukes on cattle in the UK (Sargison et al, 2016). These parasites attach by their ventral sucker to small conical papillae of the rumen and reticulum (Figure 4).
Paramphistomum infections can cause significant disease. Adult rumen flukes are mainly located in the cranial sac of the rumen and adjacent to the reticular groove. However, the clinical disease is caused mainly by the activity of juvenile flukes in the intestine of the infected host. Clinical signs include enteritis, fetid diarrhoea, anaemia and protein loss, which generates a generalised oedema (hydrothorax, hydropericardium, ascites and lung oedema).
Diagnosis is based on the demonstration of immature flukes in faeces. Faecal sedimentation to detect eggs is used. Eggs are, however, similar in shape to those of Fasciola hepatica, but slightly larger and more transparent. Paramphistomosis should be differentiated from nutritional deficiency of copper, infection with intestinal roundworms, infectious enteritides (usually accompanied by fever), Johne’s disease in adult animals (although this is much more chronic), and poisonings – including many weeds, inorganic arsenic and lead.
Infected cows can be treated with oxyclozanide. Control measures include avoidance or drainage of snail habitats, and anthelmintic treatments to prevent contamination of pastures with eggs.
The nematode lungworm Dictyocaulus viviparus is the causative agent of parasitic bronchitis in cattle. Infection with this parasite occurs worldwide, but it is of particular importance in temperate regions. The nematode has a direct life cycle and cattle are infected with the third larval stage (L3) when grazing on contaminated pastures (Elsheikha and Khan, 2011). Lungworm disease (also known as husk) causes significant economic losses due to its adverse effects on the milk production and health of affected cattle (Dank et al, 2015).
Lungworm causes parasitic bronchitis in cattle of all ages; however, D viviparus is most dangerous when encountered by a calf on pasture. In naive calves, lungworm disease can be damaging, with up to 40 per cent mortality, and survivors become stunted for the rest of their lives. After initial exposure, calves develop resistance to further infection. Clinical signs include coughing, nasal discharge, pneumonia, oedema, emphysema and inflammatory responses, which may lead to death.
Diagnosis of lungworm infection in live animals is based on clinical signs, grazing history and knowledge of the patterns of the parasite epidemiology. L1 (first stage) larvae can be recovered using faecal flotation and the Baermann technique. Tracheal washes may also provide cytologic evidence of eosinophilic inflammation consistent with parasitic bronchitis or pneumonia. Bronchoscopy and radiography may be also helpful. Two different serological tests for D viviparus infection have been described – an immunoblot-based dipstick test and ELISA based on recombinant major sperm protein.
Levamisole was one of the first drugs found to be effective against the D viviparus parasite and the benzimidazoles and macrolides are more effective in the control of lungworm disease. Treating after the migration of larvae through the lymph nodes, and before the onset of clinical signs, is more effective in preventing disease and allowing the establishment of protective immunity.
A lungworm vaccine exists made up of irradiated infective larvae. The vaccine is effective and primes immune response against any exposure to infection. Two doses are given four weeks apart, at least two weeks before animals can be turned out, to allow the development of a protective level of immunity. Vaccination against husk is a vital component of any lungworm control programme.
The economic and social impact of liver fluke infection in cattle is significant. It is indicated the prevalence of infection has increased over recent years due to climate change, increased animal movements and changing farming practices.
Liver fluke disease is often classified into three clinical forms with varying degrees of severity. These are dependent on the timing, level and duration of ingestion of infective stage metacercarial cysts. Acute fluke disease is more often seen in sheep than cattle, and occurs when animals ingest massive numbers of infective metacercariae over a short period of time.
Chronic fluke disease is the most common and widespread form of fluke disease in sheep and cattle (Elsheikha and Khan, 2011). Disease is associated with a prolonged intake of low to moderate numbers of metacercariae from herbage and results in a progressive loss of body condition associated with the accumulation of adult flukes in the bile ducts of the liver. Anaemia is often severe in undernourished animals that may also exhibit submandibular oedema (bottle jaw) – an accumulation of fluid caused by low blood protein (or hypoalbuminaemia).
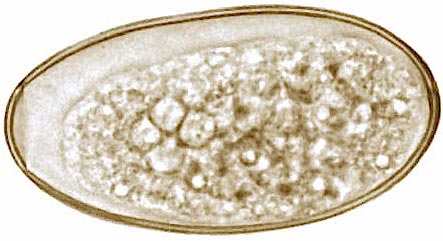
Confirmation of disease is usually through postmortem examination of the liver. In the live animal, the presence of golden-coloured, operculate fluke eggs (Figure 5) in faeces is considered as evidence of fluke infection. Blood enzyme profiles – looking specifically for liver/bile duct damage – may be of use in chronically infected animals. Also, fluke-specific ELISA tests are commercially available, which detect anti-fluke antibodies in blood and/or milk, and indicate an animal has been exposed to fluke infection.
An ELISA test capable of detecting traces of antigens released by the fluke into the host’s faeces (coproantigens) is also available. Two nucleic-acid amplification methods, PCR and loop-mediated isothermal amplification, were developed and tested against conventional diagnostic methods, such as FEC and coproantigen ELISA (cELISA). These molecular methods failed to detect F hepatica in faecal samples (Arifin et al, 2016), indicating FEC and cELISA are more sensitive and efficient in diagnosing liver fluke infection.
Control of liver fluke infection in livestock relies heavily on the strategic use of flukicidal drugs. A wide selection of such products are on the market. Oxyclozanide, nitroxynil, clorsulon, triclabendazole and albendazole/ricobendazole, at increased dose rates, have activity against adult liver flukes and are suitable for treating chronic fascioliosis. When treating cattle in late autumn and winter – a time when infections will consist of both adult and immature flukes – triclabendazole is the drug of choice, because it is also active against immature flukes.
Control of worm infection relies on an integrated approach that combines anthelmintic treatment and grazing management.
Worm infections are routinely treated with anthelmintics (wormers). The main three chemical groups of anthelmintics are benzimidazole (white drenches – group 1-BZ), levamisole imidazothiazoles and tetrahydropyrimidines (group 2-LM), and macrocyclic lactones (group 3-AV), which include the avermectins and milbemycins. Anthelmintics belonging to these groups are active against the major species of GI roundworms and lungworms. Some will also have activity against liver flukes. Macrocyclic lactones are often referred to as endectocides because they also have activity against some ectoparasites. Other products are more specific in the parasites they kill and referred to as narrow-spectrum drugs. Some are also active against certain ectoparasite species.
Anthelmintics can be administered to cattle through a specialised device, known as a bolus, which falls into two categories:
Boluses are designed for use primarily in first-year grazing animals at turnout, but can be used in cattle already at grass, or in animals in their second grazing season. Controlling parasites by selecting anthelmintics that have an effect on hypobiotic larvae allows the host to be cleared of a parasite burden before the worms do any real damage, and lessens pasture contamination before the number of larvae in the environment reach levels that may cause disease in grazing cattle.
Some products are marketed for the combined treatment of GI roundworms and liver flukes in cattle (for example, oxfendazole/oxyclozanide, levamisole/oxyclozanide, ivermectin/clorsulon and levamisole/triclabendazole); however, the rationale for using these products should be carefully assessed. Occasionally, it may be necessary to treat dairy cattle with an anthelmintic.
Wherever possible, treatments should be given during the dry period. When treatment has to be given during lactation it is important to consider milk withdrawal periods. Many of the macrocyclic lactones (with the exception of eprinomectin and fluke products containing oxyclozanide or nitroxynil) should not be used in cows producing milk for human consumption.
AR is a decrease in the efficacy of a drug against a parasitic species that was previously susceptible. The frequency of treatment is a potent source of selection pressure that occurs whenever a drug is often used. Resistant worms transmit their heritable traits to the next generation, thereby incrementally increase the frequency of their genetic alleles in the general population. The genetic basis and modes of inheritance of resistance are complex and vary widely among the various drug classes.
Resistance to anthelmintics in GI nematodes of cattle is not as prevalent as it is in sheep, but reports are becoming increasingly common for Haemonchus contortus, H placei, Cooperia punctata, Cooperia oncophora, Cooperia spatulata, and O ostertagi. AR in cattle nematodes can be detected using a FEC reduction test (FECRT).
The term refugia describes a phenomenon where a subpopulation of parasites is not exposed to the anthelmintic at the time of treatment. This includes larval stages in the environment, parasites of some individual animals left untreated, and parasitic stages in the animal not exposed to the treatment due to physiological or pharmacokinetic factors. The subpopulation left in refugia represents a reservoir of unselected genes. Refugia provides a source of susceptible worms to mate with resistant worms. When refugia is increased, the rate of resistance development will be reduced.
While strategic use of antiparasitic drugs is a valuable tool in managing the adverse impact of parasitism on animals, grazing management tools – such as the provision of clean pastures, alternate grazing by other animal species, alternate grazing by immunologically resistant hosts of the same species and monitoring of parasite transmission – can also influence the effectiveness of drug regimes.
Sound pasture management reduces the need for anthelmintics, minimises reinfection by preventing recontamination of spring pastures, and prevents the midsummer build-up of infective nematode eggs or larvae on pasture.
An effective endoparasite control programme should be designed to minimise parasite load in cattle, prevent contamination of the environment and disrupt the parasite’s life cycle. Worm control in cattle rests on the use of chemicals; however, resistance problems associated with this, and issues around the residues in animals and the environment, suggest complementary non-chemical measures should be developed and implemented.
Unfortunately, no commercial vaccines exist for many cattle worm species, except the vaccine used in the prevention of lungworm. In the meantime, the development of new anthelmintics is expensive and can take decades, thus, the effectiveness of existing anthelmintics needs to be preserved and should be supported by more frequent monitoring of worm burdens and the adoption of management practices that delay the development of resistance.
While farm animal vets play a key role in implementing endoparasite control programmes, clinical parasitologists can make significant contributions to the formulation and success of these programmes.