11 Sept 2017
Paul Wood discusses why strategies for treatment and control of coccidiosis should play an important part in herd and flock health planning.

Figure 2. VIDA data for cattle, sheep and goat submissions by month in 2015.
Coccidia have a complex life cycle that includes both sexual and asexual phases. This maximises their reproductive output, but also increases the environmental challenge they exert in clinical situations. The life cycle of Eimeria species is simplified in Figure 1.
These trophozoites undergo several divisions until they form merozoites, which can then infect further intestinal cells. Subsequent cycles of division lead to the formation of micro and macrogametes, which can fuse to form more oocysts (sexual reproduction phase). The oocysts mature, causing cell rupture, and are released into the intestinal lumen, where the cycle is completed with passage in the faeces.
UK on-farm prevalence is high, with high levels of environmental challenge occurring on many units. Adult cattle can act as passive carriers of the parasite and will contribute to the overall burden encountered by youngstock.
Inadequate cleaning and hygiene between batches of animals may also lead to accumulation of infective material, causing disease in subsequently housed groups. If cattle are grazed year round, then contamination of natural water sources can act as a focus for infection, as can the poaching of land surrounding feed and water troughs.
Oocysts can persist in the environment for protracted periods (more than 12 months) and are particularly resistant to desiccation and many disinfectants. At their peak, infected naive animals can pass millions of oocysts into their environment.
Continual low-level exposure to coccidial oocysts will lead to the development of host immunity. However, this is also species-specific (so, for example, protection against E bovis in cattle will not protect against E zuernii).
Low burdens are tolerated well by healthy animals, but shedding of oocysts into the environment will still occur in these cases, thereby contributing to the environmental challenge and endemic nature of the infection.
Coccidiosis is widespread across the world, and cases can be seen sporadically or as outbreaks. The prevalence of Eimeria species in cattle is generally high, with reports of up to 100% in some calf groups. Some studies (Stewart et al, 2008) found an overall prevalence of 7% in England and Wales. However, the prevalence and infectivity of the different pathogenic/non-pathogenic strains may be largely variable and dependent on multiple factors.
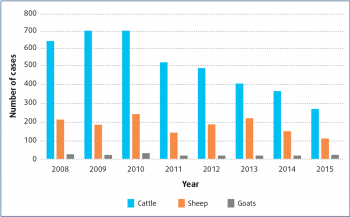
The true prevalence and/or incidence of coccidiosis in the UK is still not known. The latest Veterinary Investigation Diagnosis Analysis (VIDA, 2015), which collates diagnoses from APHA and SRUC centres across the UK, shows increases in the number of coccidiosis diagnoses reached between March and July (Figure 2). However, the year-to-year trend shows marked decreases in cases diagnosed (Figure 3). This may be due to the submission rate, but may also be as a result of accurate clinical diagnosis combined with successful early treatment and prevention.
Many factors can lead to the development of clinical disease on coccidia-endemic farms. These include housing conditions promoting increased environmental challenge or exposure (poor hygiene, overstocking) and reduced immune response (stress, poor colostrum management).
Even if calves have been exposed to low levels of disease and have developed an immunity, this immunity can still be overwhelmed in the face of a massive infective challenge. Clinical disease results from the parasite damaging the intestinal cells during its life cycle (three quarters of the life cycle is conducted within the host). This causes widespread damage to the mucosal surface and shortened villus structures.
As a consequence, absorptive surface area within the intestine is lost, and ability to absorb water and nutrients is reduced. Affected animals also show increased intestinal motility. Severity of disease is directly related to the number of infective oocysts that have been ingested, and in heavy infestations it may take as little as a fortnight for the majority of intestinal cells to be occupied by coccidial cell types.
Clinical signs generally do not appear until late in the life cycle (day 18), at which point damage has already occurred.
Clinical signs in acute disease may include:
Alongside these clinical signs, coccidiosis can also lead to immunosuppression of the individual, making it more susceptible to secondary infections. Risk of seasonal blowfly strike also increases in sheep, due to faecal contamination of coats.
In endemic farms, chronic infections may lead to significant economic losses. In growing animals, coccidiosis contributes to reduced weight gain, despite normal feed intakes, and therefore a reduction in productivity.
Infected animals will have a reduced carcase weight and/or an increased time to slaughter. Many of these animals will not show overt clinical signs, but will still be infected and shedding oocysts into the environment.
Once the parasite is endemic in a group, any susceptible animal that is introduced will be at risk of severe disease. As Figure 2 demonstrates, disease in sheep peaks over the spring months, whereas cattle can be affected throughout the year. Any housing periods of livestock may lead to increased exposure, and, therefore, peaks in disease.
Initially, a tentative diagnosis may be reached due to clinical signs and farm history. Faecal samples showing large numbers of oocysts under microscopy (McMaster technique) may indicate coccidiosis, although care must be taken with interpretation without speciation.
Large numbers of non-pathogenic coccidial oocysts may be present, but not contributing to the clinical syndrome. The presence of oocysts in faecal samples without overt clinical signs does not always necessitate treatment.
It is also important to understand that in the highly pathogenic species, severe clinical signs may be observed prior to large numbers of oocysts being detected (prepatent period for E zuernii equals 18 days; E bovis equals 17 days).
Infection with E alabamensis (which has a shorter prepatent period of eight days) is normally associated with a massive oocyst output, coinciding with clinical disease. Faecal samples can be taken from multiple animals in a group and quantitative counts interpreted together to help in diagnosis.
Definitive antemortem diagnosis is typically based on speciation of oocysts alongside suggestive clinical signs and farm history, although often the clinical picture is enough to start treatment.
Postmortem examination can reveal diffuse thickening of the caecum and intestines, mucosal erosions, haemorrhage and diphtheritic membranes. Histological examination of mucosa may reveal high numbers of oocysts, schizonts or gametes.
Initial control of disease is achieved by isolating animals showing clinical signs, as these will be a source of infection for in-contact animals. Treatment of all clinical cases with a coccidiostat oral drench should be recommended, as well as metaphylactic treatment of at-risk animals (even if they are showing no clinical signs).
Table 1 details available coccidial treatments and dose rates for use in cattle and sheep. This is a guide only, and vets should, as always, consult datasheets/SPCs for specific products.
Coccidiostats will allow immunity to develop within the animal while reducing the damage caused by infection, thus reducing the incidence of severe disease and the shedding of oocysts. This will, in turn, reduce environmental contamination.
Supportive therapy for dehydration and anorexia is also important in clinically affected animals, as the disease is often self-limiting and individuals are able to recover spontaneously if these secondary problems are addressed early. Oral or intravenous fluids and electrolytes will correct dehydration, and antibiosis should be considered if secondary bacterial infections are thought to be involved.
Injectable trimethoprim–potentiated sulphonamide preparations have been used to treat clinical cases if oral drenches are unavailable. However, antimicrobial resistance should be taken into consideration first, as bacteria (both commensals and pathogenic) will also be exposed to the antibiotic.
In-feed coccidiostats can be used to prevent disease when there is a known risk of infection on farms. This is common practice on many units where coccidiosis is a common problem.
Prevention of disease by early intervention with metaphylactic treatments can significantly affect growth rates and long-term productivity.
Preventive treatments should be given following the guidelines of the specific products, although typically this will be at least one week following the start of the risk period. Routine sampling should be undertaken to ensure preventive measures and treatments are effective/working.
Prevention of coccidiosis is essential and can only be achieved by improving management practices. As mentioned earlier, several factors contribute to increased risks of coccidiosis, so identifying these on each farm can aid in the development of a herd or flock health plan. These measures will likely include:
The significance of coccidiosis in livestock systems should not be underestimated. The disease can have a severe impact on both animal health and farm economics.
As with many diseases in livestock production, some simple improvements in management practices can have dramatic effects on reduction of disease. By understanding the life cycle of the parasite and the important role of acquired immunity, we can assist our farmers in developing effective therapeutic controls in the face of disease, and help them to understand the financial benefits of suggested improvements.