20 Mar 2017
Anna Bruguera looks at bovine viral diarrhoea, its transmission, recognising clinical signs and UK eradication schemes in place.

Figure 1. Bronchopneumonia (yellow arrows) is a common complication of bovine viral diarrhoea virus infection.
Bovine viral diarrhoea (BVD) is recognised as one of the most important diseases of cattle, and many countries, including the UK, are developing programmes to eradicate it. The virus can be transmitted through various routes, direct contact between an infected and a naive animal being the most common, but contaminated materials can also play a role in the transmission. The clinical presentations of the disease are multiple and can vary from subclinical, transient infections, to the birth of persistently infected calves that may succumb to severe mucosal disease later in life. As eradication schemes progress and the level of circulating BVD virus decreases, recognising the clinical signs and identifying routes of infection will remain crucial to promptly detecting and controlling the disease in case of an outbreak.
Bovine viral diarrhoea (BVD) is recognised as one of the most important diseases of cattle and one of the most studied due to the economic impact the disease can have in cattle production systems (Gunn et al, 2004).
BVD is in the focus of many European countries that have started eradication programmes to control it (Moennig et al, 2005). In the UK, Scotland, Northern Ireland and England have eradication schemes in place, while a scheme is being discussed in Wales.
This article presents a short review of the virus transmission and clinical presentation, as well as the structure and progress made by the eradication schemes in the UK.
BVD is caused by a virus of the Flaviviridae family, genus Pestivirus. Two species of BVD virus (BVDV) exist – BVDV-1 and BVDV-2. Both viruses have high antigenic variability, which means new subspecies and strains of BVDV-1 and BVDV-2 are constantly being discovered. So far, 16 BVDV-1 and 3 BVDV-2 subspecies have been identified (BVDV-1a to 1p and BVDV-2a to 2c; Peterhans et al, 2010).
Regardless of the species, both viruses present two biotypes – non-cytopathic (NCP) and cytopathic (CP). The CP viruses are named after their ability to cause apoptosis of cultured cells, which is due to a change in a non-structural protein (NS2-3; Kümmerer et al, 2000). CP strains are responsible for causing mucosal disease (MD) in animals that are persistently infected (PI) with BVD.
The disease has a worldwide distribution, with varying prevalences across countries. The species distribution is also variable and, although BVDV-1 is the most prevalent species worldwide, BVDV-2 has higher prevalences in the US, compared to other regions (Bolin and Ridpath, 1998). BVDV-2 is also present in Europe and it has been detected in the UK, although not in recent years (Wakeley et al, 2004; Animal Health and Veterinary Laboratories Agency, 2014).
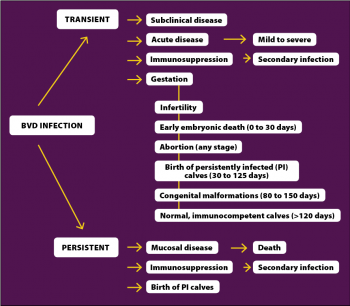
When naïve, non-pregnant cattle are exposed to the virus, they undergo a short period of viraemia that may last between 3 and 12 days (Müller-Doblies et al, 2004). These are known as transient infections. Animals may suffer from mild to severe disease, although most transient infections are subclinical. Clinical signs include pyrexia, depression, anorexia, diarrhoea and respiratory signs, which include nasal discharge and coughing. Leukopenia with lymphopenia is also associated with transient infections and, therefore, transiently infected (TI) animals become immunosuppressed. As a consequence, they may succumb to secondary infections, with respiratory disease (Figure 1) and diarrhoea being the most common complications of BVD (Bolin, 2002).
Virulent strains of BVDV are associated with severe presentations of transient BVD that, in addition to the clinical signs described, cause thrombocytopenia and mucosal ulceration, and could be confused with MD (Liebler-Tenorio et al, 2003). Severe BVD has usually been associated with BVDV-2; however, it could also be caused by BVDV-1 (Glotov et al, 2016).
If the transient infection exposed to the virus is a pregnant cow, the effects on the dam and fetus depend on the stage of gestation.
A good review of the reproductive consequences of BVDV infection is given in the article by Grooms (2004). Animals TI with BVDV develop neutralising antibodies and are clinically recovered between two and three weeks post-infection (Müller-Doblies et al, 2004). After natural exposure to BVDV, antibodies have been shown to last for at least three years (Fredriksen et al, 1999), but it is believed the immunity may be life-long.

Persistent infections are caused in utero by NCP strains of BVDV-1 or BVDV-2, approximately between 30 and 120 gestational days, before the immune system of the fetus is completely functional. As a consequence, the immune system does not recognise the virus, becoming immunotolerant to it. After birth, if the NCP-BVDV becomes CP by genetic rearrangements, or if the animal is super-infected by a CP strain, the persistent infection develops MD. MD presents with ulceration of the gastrointestinal tract, from the oral cavity (gums, tongue, cheeks and palate) to the abomasum and small intestine. Interdigital and coronary band ulceration are also common findings and these result in severe lameness. Affected animals are usually pyrexic, anorectic, depressed and suffer from severe diarrhoea that can present fresh blood and/or melena.
The presentation of MD is usually acute and animals die within a few days or weeks of onset of clinical signs (Bolin, 1995). In addition to being susceptible to MD, PI cattle have traditionally been described as having poor growth rates and being susceptible to secondary diseases, with higher mortality rates. However, many PI cattle may appear clinically normal and live for years, being able to reproduce and generate new PI calves (Figure 2).
In the past 20 years, another type of BVD infection has been described – chronic infections. There have been reports of two bulls that were clinically normal, serocompetent and BVDV antigen negative, but presented very high antibody titres against BVDV. These animals had BVDV infections localised in the testes and were able to successfully transmit the disease via AI (Voges et al, 1998; Newcomer et al, 2014).
Chronic infections may affect ovarian and testicular tissues, the CNS or circulating white blood cells. More research is needed to understand the importance of chronic BVDV infections; however, existing evidence suggests they are likely to represent a low risk of transmission of disease (Givens and Marley, 2013). A summary of the clinical presentations of BVD can be found in Figure 3.
Transmission of BVDV can be via direct or indirect contact.
Direct contact between an infected and a naïve animal is the most common route of transmission, and cattle PI with BVDV are the most important source of virus, since it is excreted in all the animal’s body secretions and fluids (Houe, 1995).
TI cattle are also able to transmit the disease, although the virus is only shed during a short period of time and the risk is much lower.
Semen from PI or TI bulls is another source of virus and cases of BVD have been transmitted through AI (Newcomer et al, 2014).
Biosecurity measures and testing protocols followed by insemination centres should guarantee the risk of transmitting the disease from commercial AI is very low, but bulls should be considered when evaluating the risk of introducing BVD in the herd.
Species other than cattle can also carry BVDV. Sheep, goats and deer have been shown to be able to transmit the disease to cattle in both experimental and field conditions (Passler and Walz, 2010). However, no evidence exists to suggest heterologus species represent an important source of the virus. A study in Spain showed although wild and domestic ruminants shared BVDV isolates, there was likely to be very little transmission between them (Paniagua et al, 2016).
In addition, in Ireland, the presence of sheep on the farm was not associated with a higher number of BVDV-positive results (Graham et al, 2013). Heterologus species may act as reservoirs, and may become important as eradication schemes progress and the levels of BVDV in the cattle population decrease.
Contaminated materials and environments can also be a source of BVDV. The virus has been shown to be able to survive in slurry for three weeks at 5°C (Bøtner and Belsham, 2012) and environments contaminated with amniotic and allantoic fluids, especially after the birth of a PI calf, can also be a source of infection (Lindberg et al, 2004).
Although their importance may be smaller, indirect routes of transmission still need to be considered, since they may become more relevant towards the end stages of eradication programmes.
In a severe BVD outbreak in Germany in 2012, human contacts (including farmers and veterinary practitioners) and vehicles were the main route of introduction of the virus to the affected farms (Gethmann et al, 2015).
During the 1990s, Scandinavian countries (Norway, Sweden, Denmark and Finland) were the first to launch eradication programmes for BVD and many European countries have followed their example (Stahl and Alenius, 2012).
In the UK, Scotland, Northern Ireland and England have eradication schemes in place (Figure 4).
The Scottish BVD Eradication Scheme was launched in September 2010 by the Scottish Government, with the support of both the veterinary and farming sectors. The scheme is based on testing at herd level and started with a stage of subsidised screening that finished in April 2011.
The first movement restrictions came into effect in January 2014 (third stage); animals identified as PI could no longer be moved or sold, all herds had to declare their status before selling breeding animals and restrictions were applied to those herds that failed to meet the mandatory testing requirements.
In June 2015, Scotland entered the fourth stage of the scheme; new legal restrictions were applied and bulk milk antibody tests were removed from the list of testing methods.
PI animals can only be moved directly to slaughter and animals from “not negative” herds cannot be moved unless they are individually tested for virus (with a negative result).
Any animals entering a herd from an untested source must be tested for virus.
Before the start of the scheme, it was calculated 40% of Scottish herds were exposed to BVD (not negative).
By the end of 2015, the prevalence had been reduced to 12.5%. In 2013 and 2014, 750 and 742 BVD PI animals were identified. In 2015, the number increased to 1,479 PI animals; however, 472 PIs were still alive by August 2016 and, as a consequence, new infections are still likely to occur.
The Northern Ireland BVD Eradication Programme started in January 2013.
Initially, the scheme was voluntary, but became compulsory in March 2016.
The Northern Irish approach is similar to the scheme in the Republic of Ireland (Animal Health Ireland).
All newborn calves have to be tested for BVD antigen, using tissue ear tags, within the first week of life and movement restrictions apply to animals that have not been tested or that do not have a negative BVD antigen result. Since the start of the scheme in 2013, and by the end of 2016, more than 880,000 animals had been tested in Northern Ireland, with 98.67% of the samples being negative for BVD antigen.
In July 2016, BVDFree was launched in England as an industry-led voluntary scheme, supported by several organisations. The scheme is based on testing at herd level with follow-up tests to identify PI animals, if required.
Advice is given on the scheme’s website regarding control and monitoring measures, and the individual animal or herd results are also available. To the author’s knowledge, no data is available about the progress made by BVDFree in England.
In Wales, the development of a national eradication scheme for BVD is being discussed. This would be similar to the schemes in Scotland and England – based on herd-testing with follow-up tests – and would start with a voluntary phase.
Certain private cattle health schemes also include surveillance and control measures for BVD, and these were available before the national eradication schemes were launched. Examples include the Premium Cattle Health Scheme, HiHealth Herdcare Cattle Health Scheme and BVD Herdcheck.
In 2012, it was calculated approximately 14% of cattle herds in the UK were enrolled in health schemes (Brigstocke, 2012). However, due to privacy and commercial constraints, BVD testing results from these schemes are rarely made available (Häsler et al, 2014).
Eradication schemes against BVD are progressing in the UK. As the level of virus decrease, veterinary practitioners will have to remain aware of the clinical presentations and potential routes of transmission of the disease, so in the case of an outbreak, infection can be detected and controlled quickly.
This article has been reviewed by Dominic Mellor BVMS, PhD, DipECVPH, MRCVS, RCVS specialist in veterinary public health, and Jayne Orr BVMS, RCVS, farm animal clinician at the University of Glasgow.