11 Jun 2018
Alexander Corbishley discusses risk factors linked to passive transfer in these animals and reveals findings of an investigation.

Figure 1. It is often assumed the more “natural“ husbandry conditions of suckler farming mean failure of passive transfer in beef calves is uncommon.
 This independent article is showcased and supported online by Vetspanel.
This independent article is showcased and supported online by Vetspanel.
Vetspanel is a panel of veterinary professionals consisting of vets, vet nurses, and practice managers who share their opinions on a variety of issues.
It is well established the neonatal calf is born immunologically naive and reliant on the passive transfer of colostral antibodies from the dam for protection against a range of infectious diseases.
The high prevalence of failure of passive transfer (FPT) in dairy calves has driven investigation into the risk factors for it in dairy herds and the widespread adoption of management strategies to optimise colostral transfer, with the mantra of “quantity, quality and quickly” now firmly established on many dairy units. Despite this, relatively little is known about either the prevalence of, or risk factors for, FPT in beef herds.
Due to the more “natural“ rearing environment of beef calves, the risk of FPT is generally assumed to be low, and while most beef producers are aware of the importance of colostrum to calf health, few consider the factors on their farm that may impact on the quality of colostral transfer.
Although it is standard advice for suckler farmers to monitor calves to ensure they have stood and sucked colostrum, very few objective measures exist that can be used to determine whether a calf has sucked sufficient colostrum within its first few hours after birth.

While udder fill, stomach fill and signs of hunger in the calf can all provide clues, in a busy pen or paddock, it can be difficult to identify calves that have not sucked. With up to one in four suckler calves in some studies failing to stand and suck within six hours of birth1, assuming “nature knows best“ could leave a significant proportion of the calf crop with partial or complete FPT.
Very few studies have been published investigating the prevalence of FPT in beef herds. In fact, almost nothing can be found in the literature for herds in the UK. Prevalence levels of FPT in excess of 20% have been reported in a number of small studies in Canada and Ireland2-6, but it is poorly understood as to the significance of FPT at a herd level under typical UK husbandry conditions. Likewise, limited information also exists regarding the risk factors associated with FPT.
In Canadian studies conducted to date, tethering, twinning and dam perinatal disease have all been identified as risk factors, although whether cow parity is a significant factor is unclear. Interestingly, maternal body condition score, while associated with calf disease, has not been linked to FPT.
One of the problems with many of these studies is different assays and cut-offs are often used, making comparisons between papers difficult. The most sensitive and specific assays are those that measure calf serum IgG concentration directly – either radial-immunodiffusion or ELISAs. These are, however, not commonly offered by commercial labs, hence the practitioner is often left using serum total protein, zinc sulphate turbidity (ZST) and gamma glutamyl transferase (GGT) as proxies of passive transfer. Of these, ZST is by far the least specific7 and while concerns are raised regarding the age of the calves at which the blood samples were taken, GGT and total protein offer the practitioner pragmatic and cost-effective ways of monitoring FPT.
The cut-offs of 8g/L to 10g/L (depending on author) serum IgG used to define total FPT in dairy herds are, however, potentially misleading, with a number of studies demonstrating suckler calves with serum IgG concentrations less than 24 g/L are significantly more likely to suffer from disease, die and have poorer daily liveweight gains8,9. These studies would, therefore, suggest – irrespective of the IgG cut-off used to define total FPT – the more antibodies the calf can absorb, the better.

Dietary restriction in the dam has been postulated to be a risk factor for FPT in suckler calves. Hypotheses for the underlying mechanism include reduced immunoglobulin yield in the colostrum, poorly developed enterocytes in the calves or some additional factor in the colostrum required for efficient antibody uptake that is missing in nutritionally restricted cows.
In the late 1970s and early 1980s, a number of feed trials attempted to examine this relationship. Unfortunately, the results were conflicting, with some studies demonstrating protein restriction in late gestation results in reduced calf serum immunoglobulin concentrations10,11, while other studies failed to identify any association12-16. The assays used in these studies varied significantly and the dietary analysis conducted was somewhat rudimentary; therefore, none have been of sufficient quality to conclusively settle the matter.
Furthermore, many of these studies involved interventions, such as milking out of the udders and tube feeding of calves from different dams, to try to investigate the different hypotheses mentioned earlier. This, therefore, means these studies are unlikely to accurately reflect what happens under field conditions. More recent feeding trials have demonstrated dietary restriction in late gestation reduces both the total yield (mass) of immunoglobulins produced in colostrum and quality of passive transfer17; however, the clinical importance of these experimental observations is unknown.
Feed is a major variable cost in any livestock enterprise and one suckler farmers are keen to control. Many spring calving herds limit winter feeding costs by relying on the suckler cow’s ability to gain condition during the grazing season, to then under-feed these cows during the housing period. While some degree of body condition loss during mid-gestation may be desirable to reduce the risk of dystocia as a consequence of excessive intra-pelvic fat, if energy and protein restriction are permitted to continue into late gestation, the concern is this restriction could have potentially damaging effects on calf viability during the first few weeks of life.
Metabolic profiling of suckler cows within the three weeks prior to the start of calving is an established method by which the energy, protein and mineral balance of the herd can be assessed18. Despite the relatively modest requirements of a suckler cow in late gestation, metabolic profiles commonly identify both protein and energy restriction in these animals. This is not surprising when looking at the ration composition, given the propensity of farmers to feed straw and other poor quality forages to housed suckler cows for the reasons discussed earlier.

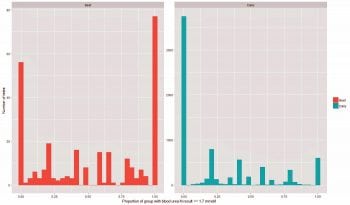
Protein restriction can be estimated by analysing blood urea-N, which provides an indication as to intakes and use of effective rumen degradable protein (ERDP) from the ration. We at The University of Edinburgh Royal (Dick) School of Veterinary Studies Farm Animal Practice19 compared the urea-N results for 2,203 beef and 39,115 dry dairy cows sampled in late gestation from across the UK, and found the mean urea-N concentration in beef cows was 1.86mmol/L, compared to 2.27mmol/L in dairy cows. This suggests ERDP intakes in beef cows prior to calving are significantly worse than those of dairy cows. In fact, in 28% of all beef herd tests, all of the animals in the group had a blood urea-N result below or equal to the 1.7mmol/L cut-off used as an indicator of insufficient ERDP. This compared to just 5% for the dairy herd tests.
We have anecdotally associated low urea-N results in cows during late gestation with an increased rate of FPT in calves, and dealt with a number of herds that have experienced high rates of FPT, where urea-N results in the cows have been low. At this stage it is unclear whether additional management or farm/cow factors (such as genetics and body condition score) are also part of the picture; however, we investigated two such spring calving herds in more detail. These herds differed significantly, with respect to size, management and genetics.
Herd one was a large (250 cow) Luing/Limousin cross-breed suckler unit. On this unit the cows were served by two rounds of AI, with conception rates approaching 70%, hence a large proportion of the herd was born within the first four weeks of the calving block. Cows were housed in large pens and fed daily using a feeder wagon and moved after 24 hours of calving into a separate nursing cow building. Stockmanship was excellent, with close veterinary involvement, excellent record keeping, detailed feed analysis and postmortem examination of all mortalities.
Herd two was a smaller unit of 75 cross-bred cows. The cows were served by a bull, with calving spread out over a number of months. Cows were fed top dressed forages and housed in the same groups until turnout. Record keeping, dietary analysis and veterinary involvement in this herd were minimal.
Both herds sought veterinary advice following an increase in calf morbidity. In herd one, 32% of the calves born in the first month of the block required antibiotic treatment, while during this time, 6% of calves younger than 30 days of age died. In herd two, while calf mortality had been negligible, the stockman had treated all the calves at birth with either penicillin or amoxicillin as the calves had seemed unusually “dull“.
Blood samples were taken from neonatal calves on both units for ZST and GGT testing, and a significant proportion of the calves in each herd had evidence of either total or partial FPT. No evidence of Salmonella or bovine viral diarrhoea was identified in either herd, while postmortem examinations from herd one identified various causes of mortality, with no single pathogen or disease syndrome predominating. In both herds, significant amounts of antibiotics were used to maintain calf health, highlighting how FPT can be an important driver of antibiotic use on farm.
Metabolic profiling of the cows indicated energy balance, long-term protein balance and mineral balance were broadly satisfactory. However, a significant proportion of the animals tested in both herds had low urea-N results, suggesting the cows were receiving insufficient ERDP to meet their needs. Herd one was fed a ration of ad-lib straw, 2kg grass silage, 5kg whole crop silage and 2kg distiller’s dark grains. Herd two was fed ad-lib poor quality haylage and 2.5kg urea-treated barley. Following the metabolic profile, both herds had their ration supplemented with 1kg of a high-protein straight (soya and beans for herds one and two, respectively). Improved calf health and reduced mortality was noted within a week of supplementation, demonstrating the rapid response to dietary supplements.
The metabolic profile results and response to intervention suggest insufficient ERDP supply in these herds was a critical factor in the quality of passive transfer in the calves. However, it is worth considering the picture is particularly complicated in ruminants, where protein and energy metabolism are, in many ways, inseparable. Both soya and beans are high in energy as well as protein, therefore it is not possible to be certain response to supplementation can be attributed solely to the improvement in ERDP supply to these animals.
It is fair to say many herds feed their cows marginal diets, with no apparent problems with calf health and mortality. However, these two herds clearly demonstrated, under some conditions, dietary restriction may have a significant impact on passive transfer and calf health and mortality. Supplementing all suckler cows with a protein-rich straight in late gestation would not be appropriate or necessarily economically justified for all herds – especially those with extended calving blocks where effects on fetal size could result in increased rates of dystocia. That said, it is clear, under certain conditions of protein restriction, additional supplementation would offer significant benefits.
We are undertaking further work to investigate the rates of FPT in beef herds under typical UK husbandry conditions, and how this may be associated with different management and risk factors, including ERDP supply during late gestation.
Thank you to Martin Tomlinson for assisting in the investigation of disease problems in the herds and Alastair Macrae for guidance in writing this article.