6 Mar 2017
Andy Durham discusses some of the commonly used approaches in treating diarrhoea cases in equine practices.

Figure 1. Strict confirmation of cyathostome emergence as a cause of diarrhoea is sometimes hard, although multiple larvae in faeces is quite suggestive.
Diarrhoea is a very common problem in adult horses. The majority of cases remain idiopathic, although diagnostic aids, such as faecal enzyme immunoassays, have dramatically improved the diagnostic rate and are worth employing. The potential contagiousness to other horses and people should always be considered at the outset when investigating a diarrhoea case. This article discusses some of the commonly used approaches in treating diarrhoea cases in equine practice.
Unlike in foals, small intestinal disease alone is unlikely to cause diarrhoea in adult horses; almost invariably, large intestinal disease will be present (alone or with small intestinal disease).
In adult horses, four general classes of diarrhoea present in practice and it is helpful to categorise cases prior to deciding the therapeutic approach. They are:
After categorising the diarrhoea case, certain empirical therapies may be applied, pending the possibility of a specific diagnosis and specific therapy. This article discusses some of the commonly used approaches to treating diarrhoea cases in equine practice.
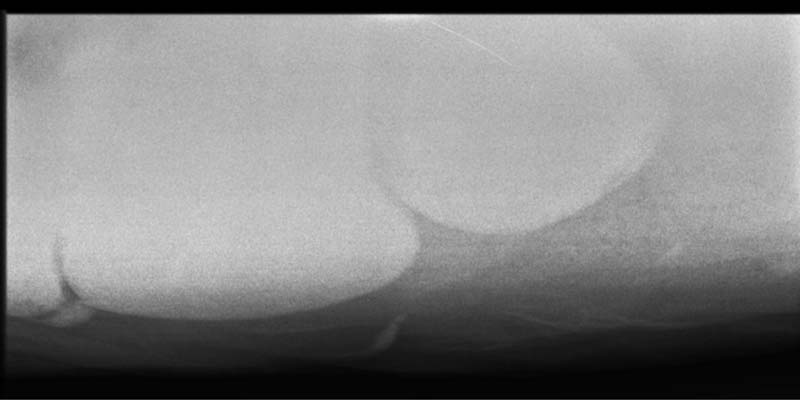
Parasitism, especially following the emergence of cyathostome larvae, is a common cause of diarrhoea and protein-losing enteropathy – especially in young horses in the winter, and commonly within a few weeks of administering an anthelmintic product (Figure 1).
The two main licensed choices for killing larval cyathostomes are fenbendazole at 7.5mg/kg to 10mg/kg po sid for five days or idectin at 0.4mg/kg po. However, the widespread benzimidazole resistance in cyathostome populations worldwide makes it hard to recommend fenbendazole, even at high and repeated doses. For all anthelmintics, a different kill rate almost certainly exists between the various larval stages and doubts exist regarding the efficacy of any product fully killing hypobiotic larvae.
The longer persistence of moxidectin is probably a key feature in its efficacy as death of more susceptible larval and adult stages may encourage emergence from hypobiosis while the drug remains at therapeutic concentrations in the circulation. Additionally, glucocorticoids can be employed to encourage larval emergence during the treatment with anthelmintics to increase kill rate.
Codeine phosphate is an effective empirical treatment of mild, non-specific diarrhoea. It may be used at an initial dose of around 0.5mg/kg to 1mg/kg bid (for example, 5 × 60mg tablets per 500kg) or up to 3mg/kg tid (25 × 60mg tablets per 500kg) in more resistant cases. Whatever the initial dose, therapy should gradually be withdrawn according to response, as abrupt cessation often leads to recurrence. For example, a good response to five tablets bid could be followed by reducing by one tablet each day.
Higher dosages require more rapid, but still gradual reductions – a good response to 25 tablets tid could be followed by reducing to 15 bid to tid on the next day and then to 10, 8, 6, 4 and so on, if there is still control of the diarrhoea. Problems with impaction are occasionally seen, but are usually manageable if detected early. Loperamide 0.1mg/kg to 0.2mg/kg bid to tid can be used as an alternative, but is more expensive.
Glucocorticoids, such as low dose dexamethasone (0.05mg/kg) or prednisolone (1mg/kg), may be indicated for a few days in persistent mild diarrhoea if codeine phosphate has not been effective alone. Chronic diarrhoea due to chronic inflammatory bowel disease carries a guarded prognosis, but a long course of prednisolone (1mg/kg sid) or dexamethasone (0.1mg/kg to 0.05mg/kg) may benefit some cases. IM injections might be worthwhile at the start of the course due to concerns over malabsorption. Treatment can be reviewed every two to three months, according to clinical response and changes in serum albumin concentrations. Treatment may have to continue for several months or, occasionally, years. Judicious doses of dexamethasone (0.05mg/kg IV) may sometimes be helpful in more severe cases of colitis in an attempt to reduce bowel oedema and inflammation. Laminitis risk should be considered in such cases.
All cases of larval cyathostominosis should be treated with dexamethasone at 0.05mg/kg to 0.10mg/kg IM daily along with larvicides. Prednisolone at 1mg/kg po sid may be a safer option and might be required for several weeks in combination with a reducing dose of codeine phosphate in some cases.
Much of the evidence suggests significant impairment of mucosal recovery by NSAIDs, so they should only be used if necessary in diarrhoea cases and, preferably, not until rehydration has been achieved due to the possibility of renal injury. Nevertheless, NSAIDs are indicated to control pyrexia and some of the effects of endotoxaemia, and may improve appetite.
Firocoxib (0.1mg/kg sid to bid IV or po) may be argued to be the preferred choice due to high cyclooxygenase (COX)-2 specificity. Meloxicam also has preferential COX-2 effects, but also has significant COX-1 antagonism. Although specific inhibition of COX-2 may be less harmful to a normal gastrointestinal mucosa, no evidence suggests it creates less interference with recovery of gastrointestinal mucosa that is already damaged or diseased.
The harmful effects of NSAIDs might possibly be countered by the prostaglandin E analogue misoprostol at 0.005mg/kg qid or even vegetable oil supplementation and this can be tried in suspected NSAID-toxicity cases.
Azathioprine may be used as an anti-inflammatory agent in horses where glucocorticoids have been unsuccessful or are undesirable due to concerns regarding adverse effects. Azathioprine appears safe to use in horses with rarely reported adverse effects, even after several weeks of use – although monitoring leukocyte numbers might be wise during prolonged therapy (suppression of leukocyte synthesis is the most likely adverse effect). Although bioavailability of the drug has been questioned, it is widely accepted as efficacious in horses at a dose of 3mg/kg sid po.
Pentoxifylline is another anti-inflammatory agent without the harmful effects of NSAIDs or glucocorticoids that can reduce several inflammatory cytokines and can be used at 8mg/kg bid po. Not many reports exist in cases of colitis, although some evidence supports its use in endotoxaemic cases.
Although better known as a prokinetic for postoperative colics, it is well recognised lidocaine offers both analgesia and also potent anti-inflammatory actions on the intestine. The author frequently uses this drug in colitis cases by constant rate infusion of 3mg/kg/hr (15ml 2% solution per 100kg/hr) after an initial “loading bolus dose” of 1.3mg/kg IV (6.5ml 2% solution per 100kg).
The use of antimicrobials in cases of diarrhoea is generally best avoided, but there are clear indications in some cases. Cases of confirmed bacterial enteritis should receive antimicrobials depending on in vitro sensitivity patterns or intuitively predicted susceptibility.
Antimicrobials with good Gram-negative potency (for example, gentamicin 7mg/kg sid IV; enrofloxacin 7.5mg/kg sid per os) may be used for aeromoniasis and salmonellosis, and oral metronidazole (15mg/kg to 25mg/kg bid per os) for enteric clostridiosis. Low dose metronidazole (5mg/kg to 15mg/kg bid PO) might also be indicated in chronic inflammatory bowel disease cases owing partially
to the suspected aetiology involving immune dyregulation and reaction against enteric microbes, and also due to the
anti-inflammatory properties of this drug.
Di-tri-octahedral smectite is perhaps the best
evidence-based intestinal adsorbent product and has been shown to neutralise endotoxin and clostridial exotoxins, and can be used in acute severe colitis cases (3g/kg initially followed by 1g/kg tid to qid) and at lower doses in mild diarrhoeas. Evidence exists of neutralised clostridial toxins and endotoxin at doses used in vivo.
A more potent toxin neutralisation effect when compared with bismuth subsalicylate has also been demonstrated. Bismuth subsalicylate is an alternative at 1ml/kg bid PO or activated charcoal at 0.5g/kg to 1g/kg sid to bid. A high incidence of gastric ulceration in diarrhoea cases has been seen – most likely as a result, rather than cause, of the diarrhoea. Nevertheless, if significant ulceration is present then omeprazole is indicated.

Fluids are a key part of managing acute and severe diarrhoea cases, but detail is beyond the scope of this article. A vital point worth emphasising at the outset is isotonic fluid therapy – in the absence of adequate plasma colloid to retain the fluid intravascularly – is potentially very harmful in hypoproteinaemic horses. Therefore, IV hetastarch, pentastarch or plasma is a key initial part of the management of protein-losing enteropathies.
Fresh plasma transfusion (Figure 2) tends to be associated with superior clinical effects in the author’s experience, although the main limitation is availability of large volumes. In practice, this can often be achieved by passing the responsibility for locating donors (preferably large geldings) to the owner of the sick horse. Personal and social media contacts often lead to a number of good donor subjects and preparation of sedimented plasma using commercially available plasma collection bags is a relatively easy procedure. Healthy donors can give around 1.5% (maximum of 2%) of their body mass as whole blood (for example, 7.5L/500kg), from which about half that volume of plasma can be separated (for example, 4L/500kg). Thus, a single 500kg donor might be expected to contribute around 140g albumin given a typical plasma albumin concentration of 35g/L. When given to a hypoalbuminaemic recipient of similar size, this might increase plasma albumin by only 1g/L to 3g/L and, therefore, multiple donors are invariably required when transfusing adult horses. Transfusion reactions are rare.
It is unlikely sodium and chloride will require specific supplementation in most cases, as these ions are plentiful in IV fluids. However, problems may arise with deficits in potassium, calcium and magnesium, as well as acid-base abnormalities. In cases of hypokalaemia in adult horses, it is generally safe to supplement Hartmann’s solution (contains 4mmol/L K+) with an additional 20mmol/L K+. This can be done by adding 10ml 15% potassium chloride per litre of Hartmann’s. The maximum administration rate of K+ should be less than 0.5mmol/kg/hr. Thus, the supplemented Hartmann’s (24mmol/L K+) should not be given faster than 10L/hr for a 500kg horse (infusion rates as fast as this are actually hard to achieve).
If hypokalaemia persists despite supplementing as aforementioned then either further potassium can be added (for example, 40mmol/L to 60mmol/L – subject, again, to a maximum rate of 0.5mmol/kg/hr) or plasma magnesium should be checked, as hypomagnesaemia may impair restoration of plasma potassium.
Hypocalcaemia is common in colitis cases and can be replaced if very low (less than 2mmol/L total Ca; or less than 1mmol/L ionised Ca2+) although some evidence shows hypocalcaemia protects against the effects of endotoxaemia. Formulae are available for calculation of supposed calcium deficits, although, in the author’s experience, they are rarely helpful or accurate. Most clinical calcium deficits in horses are correctable with 100ml 40% calcium borogluconate and this can be administered slowly IV (in fluid bag) or SC. Should this not resolve the hypocalcaemia then the dose can be repeated.
Many prebiotic and probiotic products are marketed for horses with gastrointestinal diseases and may have a reasonable evidence basis in some other species. The quality of commercially available probiotic products was scrutinised and questioned in one study, which found most products contained few, if any, viable or potentially beneficial organisms, and sometimes potential pathogens were encountered (Weese, 2002). The author has also found a pure growth of Enterococcus gallinarum, an occasional cause of antibiotic-resistant nosocomial infections in humans, in a commercially available equine probiotic product claiming to contain lactobacilli. An evidence-based approach to the design of appropriate equine bacterial probiotic products has, thus far, been unsuccessful.
The investigation of yeast-containing probiotics, including Saccharomyces cerevisiae and Saccharomyces boulardii, has provided some supportive evidence in horses with gastrointestinal disease and has been shown to protect against the adverse effects of starch overload and to significantly reduce the duration of diarrhoea in clinical enterocolitis cases. Further studies have not always confirmed this putative benefit, however, and evidence of benefit does not exist in chronic diarrhoea cases.
In human colitis cases, fungaemia has been reported in patients treated with yeast probiotics as the organism can cross a compromised mucosa, which raises concerns if these products are given to systemically sick horses with colitis. The addition of short-chain fructooligosaccharides (up to 30g daily) has also been shown to protect the colon from dysfermentative effects of carbohydrate overload.
Starch and fructan ingestion should be eliminated or restricted to ensure complete small intestinal digestion and absorption so the large bowel is not disturbed by rapidly fermented non-structural carbohydrates. To this end, temporary abstinence from grazing and exclusion of concentrate feeds – or at least limitation of concentrate feeds to no more than 1g starch per kg bodyweight (bw) per meal (for example, less than or equal to 200g to 300g concentrate feed per 100kg bw per meal) – is advisable, although such feeds could be given every four to six hours.
Additionally, a source of easily fermentable fibre, such as non-molassed sugar beet pulp, psyllium or soya hulls, may be offered along with free access to a good quality grass or alfalfa hay.
Oil should ideally be avoided until diarrhoea has resolved, then might be gradually introduced, starting at 0.1ml/kg bw per day and increasing over two to three weeks to 1ml/kg bw per day, if weight gain is considered important.
Psyllium is often fed to horses considered to be affected by, or at risk from, sand enteropathy (Figure 3) with the intention of increasing sand removal from the colon. Not all studies have supported its usefulness, but it has some evidence basis when used at 0.5g/kg to 1g/kg (this is generally much higher than the label recommendation), perhaps combined with mineral oil. Longer-term use of psyllium as a preventive can be used at the same dose rate, but is very expensive. Ultimately, dietary and management changes, such as ad libitum hay provision and restricted pasture access, may be required to control sand intake.