20 Apr 2021
Tim Mair, BVSc, PhD, DEIM, DESTS, DipECEIM, AssocECVDI, FRCVS, provides an overview of the most common types of tumours encountered in equine patients, as well as treatment options and their success rates

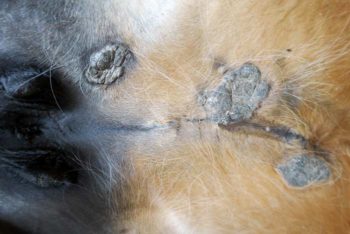
Figure 1. Occult sarcoid on the medial thigh – typically hairless, roughly circular lesions containing several small cutaneous nodules.
Fortunately, neoplasia is relatively uncommon in the horse compared to the prevalence in humans and small animals, but it is, nonetheless, a significant cause of morbidity and mortality.
Although the true prevalence of neoplasia in this species is unknown, it is generally agreed that the commonest site for neoplastic development in horses is the skin. This assumption is likely to be influenced by the fact skin neoplasms are easy to diagnose, whereas internal neoplasms (such as mesenteric lipomas) may never be recognised unless a detailed postmortem examination is performed.
In contrast to rapid advances in the treatment of neoplasia in humans and small animals, progress in developing novel treatments for neoplasia in the horse has been relatively slow. This is undoubtedly partly due to the large size and body mass of horses, which makes many therapeutic agents prohibitively expensive.
Additionally, when considering cutaneous neoplasia, the traditional approach for many years was to avoid treating skin tumours because they rarely resulted in mortality. However, now a growing population of older and geriatric horses exists, the long-term effects on the health of affected horses of this “benign neglect” attitude has become clear.
All skin tumours, however small and apparently innocuous at the time, should be taken seriously and treatment options considered to prevent longer‑term consequences.
The commonest skin tumours in the horse in the UK are sarcoids and melanomas, and these will be the focus of this article. Squamous cell carcinoma is another common skin tumour that occurs most frequently at mucocutaneous junctions, including the conjunctiva and eyelids, penis, prepuce and vulva.
Other, less common tumours can also occur, and it is not always possible to distinguish these from the commoner types on gross appearance alone. For this reason, biopsy should always be considered, especially if the behaviour or gross appearance of the tumour is not typical.
Sarcoids are locally invasive, fibroblastic skin tumours that represent the most common tumour in equids worldwide.
Recent evidence indicates an association between sarcoids and bovine papillomavirus (BPV)-1 and/or BPV-2 infection. They can affect horses, donkeys and mules, as well as zebra.
A predisposition to sarcoids has been suggested for several breeds of horses, but these studies have been based on relatively small populations and no clear evidence exists; however, genetic factors probably play a role.
Sarcoids can affect horses of all ages, but they are reported to most commonly develop between three to six years of age. Although they do not metastasise, they can significantly affect the function and appearance of the affected horse depending on their size and location. Additionally, they can ulcerate and become secondarily infected, as well as being irritated by flies (and flies are considered to be potential vectors for BPV-1 and BPV-2).
Sarcoids are classified according to their gross appearance and clinical behaviour. Six distinct types of sarcoid exist based on gross appearance and behaviour – including occult (Figure 1), verrucose (Figure 2), nodular (Figure 3), fibroblastic (Figure 4), mixed (Figure 5) and malevolent (Figure 6; Knottenbelt et al, 1995; Pascoe and Knottenbelt, 1999). However, some overlap between these types occurs, and not all sarcoids fit exactly into these groups.
Sarcoids can develop anywhere on the body, but the most common locations include the head (periorbital region, ear pinnae and lips) and neck, extremities (especially the inner thighs), and ventrum (including the inguinal region and the prepuce in males). Sarcoids may become more aggressive if disrupted by injury, biopsy or inappropriate treatment.
A definitive diagnosis of sarcoid requires histopathology, but biopsy‑induced trauma or irritation may exacerbate the lesion and induce proliferation (Knottenbelt, 2003). Therefore, a biopsy is recommended only if the diagnosis is uncertain due to atypical appearance or anatomical site, and only when owners are aware that subsequent treatment will be necessary if a sarcoid is confirmed (Taylor and Haldorson, 2013).
A wide variety of treatments can be used to manage sarcoids, but no single treatment is applicable or effective in all cases. Surgical management (including conventional excision and laser excision), cryotherapy, hyperthermia, radiotherapy, chemotherapy, immunotherapy, topical immune modulation and antiviral agents have been used with variable degrees of success.
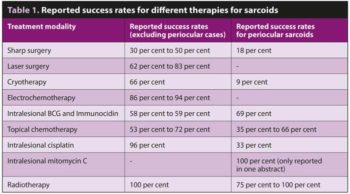
Sarcoids in particular anatomical locations can be especially difficult to treat, such as periocular sarcoids and sarcoids in the axilla/olecranon region. A summary of the more commonly used treatments and some of their reported success rates is shown in Table 1.
Conventional surgical excision may be possible in some cases, but it is essential to establish wide surgical margins (2cm to 3cm) to prevent recurrence due to inadequate removal of all sarcoid tissue in the surrounding tissue. Recurrent tumours are often aggressive and regrow more rapidly than the initial tumour (Tarwid et al, 1985; McConaghy et al, 1994). For these reasons, traditional sharp excision of sarcoids is not generally recommended.
Surgical lasers have advantages over conventional scalpel blades because they cut and vaporise soft tissue, and are less likely to spread sarcoid cells as they cut. Laser surgery also causes less haemorrhage, and less postoperative oedema and pain (Palmer, 1996). CO2 lasers (Figure 7) are ideally suited to skin surgery and have some advantages over diode lasers in terms of the speed and precision of cutting tissue, with less collateral damage.
Cryotherapy involves the application of liquid nitrogen at -196°C, either by spray or probe, to destroy tumour cells through the formation of intracellular ice and subsequent rupture of cell membranes (Figure 8). Sloughing of tissue will occur two to four weeks after cryotherapy, and tissue depigmentation may remain for six or more months.
Repeated treatments may be required for large or recurrent lesions, and post‑treatment complications may include collateral damage to nearby tissue and extreme scar contraction. Tumour recurrence is more likely for periorbital sarcoids (Knottenbelt and Kelly, 2000) because achieving full freezing in this area may be more difficult.
Radiotherapy uses ionising radiation to kill neoplastic cells by damaging DNA and protein. Brachytherapy uses a small, sealed, radioactive source implanted within – or placed on – tumours to allow high doses of radiation to be delivered for a given radiation dose. These implants are often placed into the tissue after surgical debulking during anaesthesia and remain in the tissue for approximately three weeks.
Recently, a technique using high‑dose rate brachytherapy has been described with a reported success rate of 100%for periocular sarcoid treatment (Hollis, 2019). Availability of this form of treatment is limited.
Electrochemotherapy (ECT) is a recent therapy for equine sarcoids that uses electrical field pulses to increase cell membrane permeability and, therefore, increase cisplatin or bleomycin delivery to the tumour (Figure 9).
A retrospective analysis of 48 sarcoid-bearing equids treated with cisplatin ECT alone, or in combination with surgical excision, yielded a four‑year non‑recurrence rate of 98% (Tamzali et al, 2012).
The downside of ECT is that it requires general anaesthesia, and several treatment sessions may be required depending on tumour size, location and depth of infiltration.
Several forms of chemotherapy, both topical and intralesional, have been used to treat sarcoids with variable success.
Five‑fluorouracil (5-FU) is a topical chemotherapeutic drug that inhibits DNA synthesis, and has been used both topically and intralesionally. Intralesional injection of 5-FU every two weeks for up to seven treatments resulted in complete resolution of sarcoids in 61% of horses for up to three years (Stewart et al, 2006).
AW3/4-LUDES are compounded topical chemotherapy creams that contain 5% fluorouracil, heavy metals and thiouracil. They are caustic agents that are dispensed at varying concentrations, and cause inflammation and necrosis of sarcoid tissue without harming normal skin (Knottenbelt and Walker, 1994). Application of the cream daily, or every other day, is recommended for three to five treatments, with necrosis of the sarcoid expected 5 to 10 weeks after the final treatment.
Intralesional injection of mitomycin C has recently been investigated, but few reports exist of its efficacy.
Topical creams that contain bloodroot and zinc chloride are claimed to stimulate the local immune response to activate killing of tumour cells. They have been anecdotally reported to successfully treat sarcoids, but no controlled studies are available.
Imiquimod, an immunomodulator drug, has also been used topically to treat sarcoids. In one small study, 60% of sarcoids completely regressed after treatment with 5% imiquimod cream (Nogueira et al, 2006). Complications included alopecia, erythema, erosions and depigmentation of the tumour and periphery.
Melanoma is a common, variably pigmented (grey/ brown/black), infiltrative neoplasm commonly seen in grey‑coated and white‑coated horses. According to one widely cited reference, virtually all grey horses will develop “melanocytosis” if they live long enough (McFadyean, 1933).
Debate has occurred over the years about whether melanomas should be classified as benign or malignant neoplasms; currently they are generally considered to be malignant, or at least neoplasms with malignant potential (Moore et al, 2013).
Valentine (1995) proposed that four manifestations of equine melanotic disease exist – melanocytic nevus, discrete dermal melanoma, dermal melanomatosis and anaplastic malignant melanoma. Discrete dermal melanoma and dermal melanomatosis – collectively referred to as dermal melanoma – are the commonest types.
Dermal melanomas are most commonly seen in mature horses. Discrete dermal melanomas generally exist as single masses in typical locations – including the under surface of the tail, anal, perianal and genital regions, perineum, and lip commissures.
Dermal melanomatosis is characterised by multiple cutaneous masses, with at least one of the masses presenting in a “typical” location (Figure 10). Horses with dermal melanomas may also develop melanoma in extracutaneous sites, including the parotid glands and subauricular lymph nodes (Figure 11).
Melanomas may persist for many years and cause no clinical problems for the horse. However, this can change, with the lesions enlarging and coalescing. In some cases, coalescing perianal tumours can become large enough to limit defecation and result in faecal impaction (Figure 12). Lesions may ulcerate and develop necrotic cores, leading to secondary bacterial infection.
Deeply infiltrating melanoma may compromise the blood supply, leading to the development of fissures penetrating deeply into muscles and bone (Figure 13). Metastasis via the bloodstream and lymphatics can also occur, leading to tumour growth in internal organs.
Treatments for melanoma depend on their size and location. Surgical excision (including laser surgery) can be very effective when performed on early tumours on the tail or in the perineal region (Figure 14; Groom and Sullins, 2018). Development of new dermal melanomas after surgical removal is, however, possible. Cryotherapy can also be used to treat dermal melanomas.
Surgical excision is not feasible for advanced cases, particularly those with significant invasion of tumours in areas such as the parotid salivary gland.
Cimetidine, a histamine type‑two receptor antagonist, has been recommended as a therapeutic agent for melanoma (Goetz et al, 1990); however, later studies failed to support its use in most cases.
Intralesional cisplatin, using a sesame oil‑based formulation with added epinephrine, has been reported to be beneficial in the treatment of equine dermal melanoma (Theon et al, 2007). Also, implantation of cisplatin‑containing biodegradable beads has been shown to be effective, either alone or following surgical debulking of dermal melanomas. In one study, this treatment resulted in “successful resolution of the tumour for at least two years” (Hewes and Sullins, 2006).
Intralesional injection of mitomycin C has also been reported, but little published information exists of its efficacy for treating melanomas.
Melanoma is a highly immunogenic tumour, and various types of vaccines have been assessed for treatment. The canine Oncept vaccine contains plasmid DNA‑targeting tyrosinase, a glycoprotein essential for melanin synthesis and demonstrated to be overexpressed in melanomas. This vaccine has been used to treat melanomas in horses, but results are variable, with tumours shrinking in some cases, stabilising in some and apparently not responding in others.
Some drugs mentioned in this article are used under the cascade.