17 Nov 2014

Figure 2. Wound soaker catheter anchored to skin.
Recognition and quantification of pain in horses is notoriously difficult due to a lack of validated pain scoring systems and difficulty in interpretation of equine pain-related behaviour.
Many attempts have been made to objectively assess pain in animals – much of the literature is replete with studies aimed at quantifying pain in small animals. While many pain-related behaviours are described in experimental horses (for example, electrical or thermal stimulation of the coronary band; induced visceral and synovial pain), interpretation of clinical pain is much more difficult.
As the horse is a prey animal, the display of pain is often minimised, except when severe (for example, colic pain). Equine pain scales do exist, but global scales, such as numerical rating scales and visual analogue scales, are subject to poor inter-observer agreement (Holton et al, 1998; Ashley et al, 2005) and can be insensitive to mild to moderate pain (Seymour, 1982).
Objective, reliable and validated measures of pain for horses are scarce. However, developments of composite pain scales have been reported (Bussières et al, 2008; Van Loon et al, 2010; Sutton et al, 2013). While these scales are an improvement and demonstrate better predictive qualities, they still require further refinement and application within clinical settings. A reported “Grimace Scale” for post-castration pain in horses may prove promising when assessing this type of acute pain (Dalla Costa et al, 2014).
The equine practitioner must be familiar with the legislation that governs the proper use of medicines in veterinary patients. Information on pertinent legislation can be found using the VMD website. The prescribing of appropriate analgesic drugs to horses can be difficult due to the limited number of licensed products, although considerably more have become available in recent years. However, for difficult to manage pain, use of the prescribing cascade is necessary.
In the EU, horses may be destined for the human food chain and it is imperative the equine practitioner abides by this legislation. Before prescribing any medicine to a horse, the keeper must provide the passport for that animal and the veterinarian must be sure the passport provided relates to the horse being treated. Section IX of the passport should be completed as this states whether the horse is to enter the food chain. If Section IX is not signed then the default position is the horse will be entering the food chain.
Prescribing medicines – particularly analgesic drugs – becomes more difficult if the horse is destined for consumption. Use of the Allowed Substances Table of Commission Regulation 37/2010 and the Essential List for Horses is necessary and minimum withdrawal times must be applied.
For emergency treatment of injuries where the passport is not available, which will undoubtedly include the administration of analgesic drugs, a list of those drugs and a statement indicating if the horse can enter the food chain must be provided to the keeper. It should be noted administration of phenylbutazone to horses necessitates withdrawal from the food chain.
Further complicating matters, the equine practitioner treating horses entering affiliated events – such as affiliated British Showjumping Association competition, British Horseracing Authority races and Fédération Equestre Internationale (FEI) competitions – must avoid the administration of certain medicinal products. Many substances thought to have a performance enhancing effect are banned from use or are controlled in competing horses. The FEI website lists a comprehensive table of these substances.
Keepers of horses that test positive for banned or controlled substances are liable to substantial fines and suspension of the horse from competition. Veterinary surgeons involved, knowingly or unknowingly, may be liable for RCVS disciplinary action, be struck off the register and may also face substantial fines if found guilty.
Although NSAIDs have formed the foundation of analgesia in horses for many years, it is now generally accepted other drugs and routes of administration may be useful for pain management in this species.
The terminology of analgesic strategies includes:
• Pre-emptive analgesia – the administration of analgesics prior to the initiation of noxious stimulation (for example, surgical procedure), which is thought to be more effective than initiating analgesic treatment after injury. This may reduce immediate postoperative pain, and development of chronic pain states may be prevented (Woolf and Chong, 1993).
• Multimodal analgesia – combines different analgesics that act by different mechanisms and sites within the nervous system. Synergism may occur, resulting in improved analgesia and dose reduction of individual drugs. Benefits include the minimisation of drug side effects.
• Preventive analgesia – aims to block the development of sustained pain. A preventive analgesic regimen is one that employs both pre-emptive and multimodal strategies, and continues into the early postoperative period (Minghella and Auckburally, 2014; Bussières et al, 2008).
Using these strategies, it is more likely pain will be managed effectively, lower doses of analgesics can be used and side effects may be reduced.
Horses experiencing acute pain may be managed with traditional analgesics such as NSAIDs and opioids, which have been comprehensively discussed elsewhere in the literature. Adjunctive analgesics – also called analgesic adjuvants – are drugs that have primary indications other than pain (Lamont and Mathews, 2007). They also have the potential to improve comfort without adverse effects such as sedation, gastrointestinal tract dysfunction, respiratory depression or renal toxicity, often associated with traditional analgesics (Wagner et al, 2002; Wagner et al, 2010; Lamont, 2008). These agents are commonly – but not exclusively – co-administered with traditional analgesics to provide “multimodal analgesia”.
Gabapentin was first described in humans as an anticonvulsant and for treatment of neuropathic pain. It is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid and has the potential to produce anxiolytic, analgesic, sedative, muscle relaxant and anticonvulsant activities in horses, similar to those of benzodiazepine and barbiturates (Dirikolu et al, 2008).
Its mechanism of action is unclear, but involves inhibition of calcium channels and subsequent reduced neurotransmitter release. The pharmacokinetics of gabapentin have been described in the horse following oral and intravenous administration (Dirikolu et al, 2008).
Gabapentin has a relatively poor bioavailability in horses after oral administration compared to other species. Evidence of its efficacy is lacking, but the administration of gabapentin (2.5mg kg-1 PO q 12 hours) as an adjunct to traditional pain therapies was reported to have improved the signs of pain associated with femoral neuropathy in one horse (Davis et al, 2007).
Tramadol is a synthetic, centrally acting analgesic with weak mu-receptor agonist activity and inhibition of synaptic re-uptake of serotonin (Kukanich and Papich, 2009). There is little information on its administration to horses. Doses exceeding 2mg kg-1 to 3mg kg-1 result in adverse reactions – tremors and head nodding – although its half-life is short following IV administration (one to 1.4 hours; Kukanich and Papich, 2009).
The oral bioavailability of tramadol in horses is very poor; this route of administration is unlikely to be effective in horses (Shilo et al, 2008). Tramadol alone produced limited analgesia in horses with chronic laminitis, but when administered with ketamine the combination significantly enhanced the analgesic effect of tramadol (Guedes et al, 2012).
Paracetamol is a phenolic compound used for short-term treatment of mild to moderate pain. It is a weak inhibitor of cyclo-oxygenase enzyme isoforms and its analgesic action may also involve serotonergic and cannabinoid pathways (Pickering et al, 2006). It is available for veterinary use in combination with codeine, but there is very limited information regarding the use of paracetamol in horses.
The oral bioavailability of paracetamol in horses is 91 per cent compared to 45 per cent in dogs (Neirinckx et al, 2010). One case report (West et al, 2011) reported the use of paracetamol using an oral dose of 20mg kg-1 in conjunction with phenylbutazone to control laminitic pain in a pony.
Ketamine is a dissociative anaesthetic, but is thought to produce an anti-hyperalgesic effect at subanaesthetic doses (Abrahamsen, 2007; Coetzee et al, 2010) due to its N-methyl D-aspartate receptor antagonism.
Occasionally, horses with moderate to severe colic pain refractory to standard analgesics may respond to the administration of low dose (micro dose) ketamine. This “ketamine stun” can alleviate pain when used in combination with low doses of alpha2 agonists such as xylazine or detomidine. Doses of 0.2mg kg-1 IV repeated every 15 to 20 minutes may facilitate examination of the patient and provide adequate analgesia for up to two hours prior to surgical intervention (Abrahamsen, 2007).
Background sedation with an alpha2 agonist is necessary to prevent CNS excitation. Short-acting alpha2 agonists should be topped up as necessary. The short duration of action prevents clinical signs being masked if surgical intervention is necessary. It should be noted ketamine and its metabolite nor-ketamine can accumulate and may lead to recumbency.
Ketamine may also be useful administered as an infusion at subanaesthetic doses (2μg kg-1 min-1 to 10μg kg-1 min-1) for the treatment of chronic pain states.
Although widely administered to companion animal species as part of an analgesic regimen, there are few reports of the administration of ketamine infusions to conscious horses in clinical practice (Guedes et al, 2012).
Wound soaker catheters (WSC) are thin, flexible, hollow tubes with holes arranged along the length (Figure 1).

The catheter is sutured into the deepest part of the wound, with the injection port anchored to the skin (Figure 2).
Intermittent injection or continuous infusion of local anaesthetic (for example, bupivacaine) may be administered. This technique is frequently used in small animal patients and is an alternative means of providing local anaesthesia in postoperative patients (Abelson et al, 2009).
Zaccuro et al (2007) reported a technique for sustained pain relief in the lower forelimb of horses using a continuous peripheral nerve block via a WSC. Minghella and Auckburally (2014) reported that satisfactory analgesia was achieved in a mare undergoing bilateral rostral mandibulectomy by administration of local anaesthetic into the surgical wound via a WSC. This technique was used as part of a preventive multimodal analgesic plan.
Transportation of painful horses due to colic or a fractured limb can be challenging. Careful consideration regarding the provision of analgesia to these patients is necessary – especially if there is the possibility of the horse going down in the transport box/trailer and causing further injury.
The duration of travel is an important factor when considering the most appropriate therapeutic option. For short distances, adequate analgesia may be achieved with a single dose of an NSAID and an alpha2 agonist of moderate duration such as detomidine. Note – administering analgesics to horses with colic may impair diagnosis at the referral hospital. However, welfare of the horse during transportation is paramount.
Owners may be instructed to administer further medication if transport time is expected to be long and additional analgesia is required. This may carry significant risks to the owner as entering a trailer with a painful horse can be dangerous. Some veterinarians may choose to travel behind the trailer to administer further medication.
When transporting painful horses for long distances, infusions may be extremely useful. For patients experiencing moderate to severe pain, intravenous infusions of drugs listed in Table 1 can be administered.
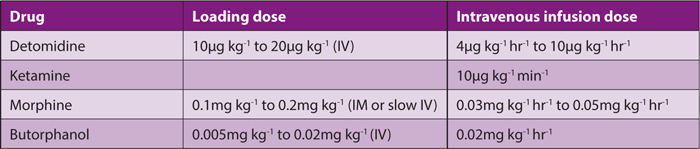
Detomidine (or an alternative alpha2 agonist) can be administered with ketamine by infusion, adding an opioid if pain is severe.

The use of an elastomeric pump (Figure 3) to administer the infusion may be considered. This will prevent changes in dose administered during transportation if the horse becomes recumbent. Elastomeric pumps are used to administer a variety of drugs at a continuous rate without the need for expensive electronic syringe drivers or fluid pumps. The pump is not gravity driven and therefore it does not matter if the patient becomes recumbent during administration of medication. The rate of delivery is determined by the infusion line attached to the pump and is constant throughout delivery until close to the end of the infusion.
These devices can be extremely useful for the transportation of painful horses to a referral establishment, since many “one-shot” analgesics are short-acting. They can be filled with a variety of drugs and attached to the horse using an elastic surcingle to provide continuous analgesia.
Transdermal fentanyl (patches) has been used in horses, although there is a lack of evidence of its efficacy in this species. Peak plasma concentrations are prone to wide individual variation (Orsini et al, 2006). Adherence of the patch to the skin is often difficult due to the presence of hair or skin debris, possibly resulting in variable fentanyl uptake.
A transdermal fentanyl solution with a novel transport system has been licensed for use in dogs. It is applied as a “spot-on” and has been shown to provide reliable analgesic plasma concentrations of fentanyl for up to four days. Experiences using this drug in clinical canine cases have been extremely positive, although there are some safety considerations when handling animals following application. This formulation may become adapted for use in horses in the future.
Variations in human genetic makeup exist that alter responses to mu opioid analgesics. Opioid pharmacogenetic variation is implicated in the variable effects of tramadol, fentanyl, codeine, morphine and methadone in humans. This may affect outcomes of pharmacological studies and skew data leading to misinterpretation of trials. In the future, personalisation of opioid therapy for individual patients may result in better analgesic management (Argoff, 2010). Although there is no evidence for this in horses, opioid switching or rotation could be considered if a particular opioid appears to be poorly efficacious or leads to unacceptable side effects.
The diet of horses is often supplemented by the owner or keeper with a variety of herbal products. Herbal remedies are subject to far less regulation than medicinal products, despite many of these supplements having the potential to interact with analgesic drugs.
In particular, NSAIDs may interact with herbal supplements known to have anti-platelet effects (gingko, garlic, ginger, bilberry, meadowsweet) and increase the risk of bleeding; Echinacea and meadowsweet may increase the hepatotoxic potential of paracetamol; valerian and chamomile may interact with opioids leading to increased CNS depression; and ginseng may inhibit the analgesic effects of opioids (Abebe, 2002).
Pain management in horses can be challenging and there is a lack of evidence of efficacy for many adjunctive analgesic drugs, although clinical experience and familiarity is increasing. As new drugs and techniques become available for other species, adjusting these for use in horses may offer the practitioner more options to deal with difficult to manage pain syndromes in the horse.
Table 1. Suggested doses of analgesic drugs when administered by constant rate infusion. Note the units for each drug.