25 Jun 2018
Sarah Smith and Veronica Roberts aim to clarify where the latest research can help us and aid in highlighting emerging threats, and they also cover imaging innovations.

Diagnostic options for neurological disease in equine patients have advanced significantly in the past few years.
The available options for imaging the head and neck continue to progress in both capability and image quality, with improved ability to image the cranial nerves and cervical spinal canal. This has improved our understanding of the anatomy of the cranial nerves and significant advances have also been seen in our understanding of the pathogenesis of trigeminal-mediated head shaking.
Alongside this, increased availability of travel for horses and the spread of vector-borne diseases with climate change is increasing the risk posed by infectious neurological diseases, in particular West Nile virus (WNV). Ongoing research has also improved our understanding of our diagnostic capabilities for infectious diseases, such as Lyme disease, where accurate antemortem diagnosis remains challenging.
Cerebrospinal fluid (CSF) sampling can be used to aid the diagnosis of a range of neurological conditions, such as trauma, meningitis and equine protozoal myelopathy.
In practice, samples are usually collected from the lumbosacral space under standing sedation. However, a discrepancy can be seen in the cytological appearance of CSF between samples obtained from the cerebellomedullary cistern and lumbosacral space. For this reason, it is often desirable to obtain a sample from the atlanto-occipital region requiring general anaesthesia; however, many neurological patients are not good candidates for general anaesthesia.
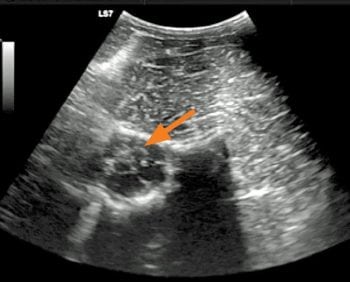
Clinicians at Michigan State University have described obtaining cerebrospinal fluid samples from between the first and second cervical vertebrae in the standing horse under ultrasound guidance (Pease et al, 2011). The described technique uses a ventral to dorsal approach and ultrasound guidance to avoid nerve roots and vertebral vessels (Figure 1).
The subarachnoid space is approximately 0.5cm in height at the level of C1-C2, although the authors report no adverse reactions, at the time of sampling, or reported complications. This approach to the subarachnoid space at the level of C1-C2 was also used to perform myelography, under general anaesthesia, in a single patient with structural abnormalities of the atlanto-occipital space (Aleman et al, 2014).
Myelography is commonly used as a diagnostic procedure in ataxic horses to identify cervical spinal canal stenosis. The standard technique currently requires introduction of contrast medium into the subarachnoid space via the dorsal aspect of atlanto-occipital space. This is performed under general anaesthesia. Anecdotal reports of attempting positive contrast myelography in the standing patient at the level of C1-C2 describe a high risk of seizures, and the technique is not recommended.
“Wobbler’s syndrome” (cervical vertebral malformation/stenosis) is the most common cause of ataxia in the UK and characterised by narrowing of the cervical spinal canal.
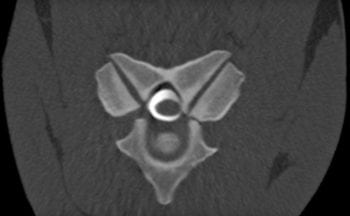
Evaluation of the complex anatomy of the equine cervical spine is notoriously challenging, particularly accurately identifying sites of cervical spinal canal stenosis. Cervical radiography and positive contrast myelography have, until very recently, been the gold standard antemortem diagnostic tests for cervical spinal canal stenosis (Figure 2).
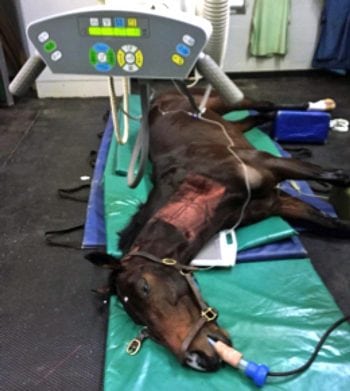
The myelography images were used to assess cervical canal stenosis in the sagittal plane by measuring the height of the dye columns dorsal and ventral to the spinal cord. A major limitation is lateral compression is not usually detected. However, interest is increasing in the use of MR imaging and contrast-enhanced CT imaging of the equine cervical spine.
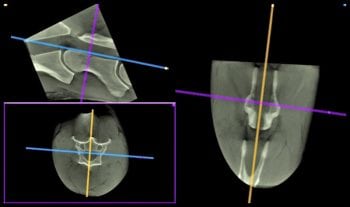
The advantage of these modalities is they allow three-dimensional imaging of the cervical spine and assessment of stenosis of the spinal canal (Figure 3). Latest studies have shown vertebral canal area and cord-canal area ratio provide a more accurate assessment of spinal canal stenosis than can be obtained from conventional radiographic myelography (Janes et al, 2013; Sleutjens et al, 2014).
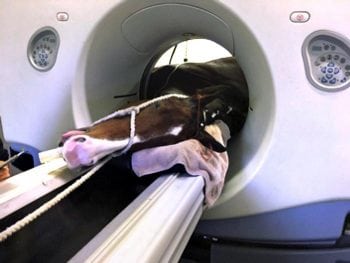
At present, MR images of the entire cervical spine can only be acquired postmortem. However, it is possible to perform CT imaging and contrast-enhanced CT imaging of the cervical spine antemortem under general anaesthesia. Systems are also available to perform CT imaging of the cervical spine in the standing horse (Figure 4).
Papers have described imaging of the skull foramina, and at some points the cranial nerves on CT images (GonÇalves et al, 2015) and high-field MRI, which allows better imaging of the nerve bundles (Dixon et al, 2017). However, in live horses it remains challenging to identify all 12 cranial nerves.
Trigeminal-mediated head shaking is an idiopathic, neuropathic facial pain condition of the horse. The condition can have significant welfare implications (Pickles et al, 2014). Anecdotally, awareness among horse owners that the condition could be medical, rather than behavioural, in its origins is increasing. Despite this, while owners reported 4% of the UK equine population showed signs of head shaking, veterinary help had been sought for only 1% (25% of those showing signs; Ross et al, 2018).
Diagnosis of trigeminal-mediated head shaking is made based on a high clinical suspicion, alongside exclusion of other causes. Without a definitive test, misdiagnosis is always a risk.
The latest advance in our knowledge of trigeminal-mediated head shaking is the discovery the trigeminal nerve of affected horses is sensitised, having a reduced threshold for activation, thought to result in neuropathic pain (Pickles et al 2011, Aleman et al 2013).
The activation threshold is tested using a technique called somatosensory evoked potentials. This is a complex procedure, performed under general anaesthesia. At present, we require more data to see if the reduced threshold is a consistent finding, between and across individuals, including determining if the threshold level returns to normal in seasonally affected horses when they are out of their season for showing clinical signs.
Once this data has been collected, however, it may be possible to use somatosensory evoked potentials to give a definitive diagnosis of trigeminal-mediated head shaking.
EquiPENS neuromodulation (Figure 5) is the safest and most efficacious method of managing trigeminal-mediated head shaking in cases that do not respond to a nose net (Roberts et al, 2016 and unpublished data). It is thought, although certainly not known, the technique may return the threshold potential to normal.
Should the use of somatosensory evoked potentials become routine for diagnosis, a role may exist for them to help individually tailor neuromodulatory treatment.
The global importance of WNV is creeping closer to home. WNV is the most widespread member of mosquito-borne flaviviruses. The virus has an endemic cycle between birds and mosquitoes, but can spill over to infect humans and horses, with the potential for large-scale morbidity and mortality.
Most infected horses will not show clinical signs; however, approximately 10% will show signs of encephalomyelitis with altered mentation, cranial nerve and spinal cord function. Approximately one third of clinically affected horses die or are euthanised.
Seasonal (late summer) outbreaks of disease in humans and horses associated with WNV infection have been reported for decades in southern and eastern Europe. The disease is thought to be endemic in several countries, including Italy, Hungary and Greece. The APHA (formerly AHVLA) has been carrying out surveillance in birds since 2001 and tests samples from horses for IgM, total antibody, virus-neutralising antibody and virus detection.
The APHA screening programme has not yet identified WNV in any birds tested. As WNV is a notifiable disease in horses in the UK, suspicion of equine encephalomyelitis is notifiable. However, due to the importance of surveillance for WNV in equids it is possible to submit samples to rule out WNV as a differential diagnosis without invoking the restrictions that would be associated with a notifiable disease outbreak.
No cases of WNV infection have so far been reported in horses indigenous to the UK. However, a case report was made of suspected West Nile encephalitis.
A horse imported from Cyprus via road and sea across Europe developed signs of encephalitis three days after arrival in the UK (Fooks et al, 2014). Blood was tested by the APHA for WNV serology and virus identification was seropositive (total antibody positive and low positive for IgM antibodies). Current viraemia was not evident by the time of testing and, therefore, WNV could not be confirmed as the cause of disease in this case. The authors of this case report speculated the horse was exposed to the virus prior to entering the UK.
The mosquito Culex modestus is considered to be the principal bridge vector of WNV as it feeds on both mammalian and avian hosts. Until 2010 it had not been identified in the UK, although studies in recent years identified populations of C modestus throughout Kent and Essex. The presence of an established vector population, along with migratory birds, increases the risk of WNV incursion into the Thames estuary (Cull et al, 2016).
The association between Borrelia burgdorferi and Lyme disease in the horse remains poorly understood. Infection with B burgdorferi occurs commonly in horses, but most seropositive horses are clinically normal (Funk et al, 2016).
Studies in Virginia used the Lyme multiplex assay (a multiple antigen immunofluorescent assay that detects antibodies against three outer surface proteins of B burgdorferi) and identified seroprevalence of 33% in clinically normal horses presented for blood sampling for other reasons (Funk et al, 2016). Follow-up testing showed no significant difference in the test results five to 17 months later.
Neuroborreliosis is an important, but rare, cause of neurological disease in horses. Clinical signs are non-specific and do not occur in every case. A series of 16 cases of neuroborreliosis were confirmed at postmortem examination; clinical signs had included weight loss/muscle atrophy, cranial nerve deficits, ataxia, changes in behaviour, dysphagia, fasciculations and neck stiffness, which occurred in several cases. Other signs occurred less commonly, such as uveitis, fever and joint effusion (Johnstone et al, 2016).
Neuroborreliosis is difficult to diagnose and postmortem histology is the gold standard. Researchers at the University of Pennsylvania investigated whether using the Lyme multiplex assay on both serum and CSF to create a CSF:serum ratio would allow differentiation of horses with neuroborreliosis from other neurologic diseases. However, positive Lyme multiplex results were common in horses with neurologic disease and did not adequately differentiate horses with neuroborreliosis from other diseases.
Not all horses with a postmortem diagnosis of neuroborreliosis had positive serum or CSF results and only 3/10 had a CSF result four-fold higher than the corresponding serum result.
Results were similar for the 70 horses used in the study that had other neurologic diseases (Johnson et al, 2018). At present, diagnosis of neuroborreliosis remains challenging due to the very varied clinical presentation and the lack of sensitive and specific diagnostic tests.
Diagnosis and management of neurological disease in an animal as large as the horse is likely to always be challenging. However, as practitioners we must be aware the field is moving forward to allow us to make more accurate diagnoses and, in some cases, treatment with less invasive methods.
With climate change and increased worldwide travel of horses, we must remain vigilant for potentially catastrophic diseases new to the UK.