31 Aug 2018
Is an objective evaluation of a ‘sore backed’ horse possible?
Andy Fiske-Jackson discusses whether equine practitioners are able to subjectively assess horses that present with "sore backs" by looking into various assessments of pain.

Figure 1. Inertial measurement units in place to measure back (thoracolumbosacral) movement
Equine veterinary practitioners will frequently be presented with a horse deemed to have back pain or dysfunction.
This may be due to signs the horse is exhibiting – for example, bucking, rearing, sensitivity to brushing or on palpation, or following assessment by a physiotherapist, chiropractor or osteopath.
A reaction to pressure over the spinous processes, or over the epaxial musculature, can often be elicited. Pressure algometry has been shown to provide an objective measure of the pressure required to elicit a pain response – the mechanical nociceptive threshold (MNT) – with reference MNTs within the axial skeleton of normal horses documented (Haussler and Erb, 2006). A reduction in the MNT was shown to correlate with sites of induced back pain.
Handheld devices are certainly a rational tool for equine practitioners during examination of the horse’s back. However, most clinicians would appreciate horses show a wide variation in sensitivity to back palpation, which appears to be as much associated with breed – for example, cob versus Thoroughbred – as potential sites of pain. Pressure algometry is potentially more useful in assessing a response to treatment with serial examinations, with the horse acting as its own control.
Alternatively, attending vets may be presented with a horse considered to be performing below its potential and, following clinical examination, back pain or dysfunction will be on the list of differential diagnoses. The small range of movement of the back, and the difficult accessibility, mean routine diagnostic procedures used in lameness examinations are unsuitable and insensitive for assessing back pain.
If a suspicion of back pain has been raised, clinicians must prove it. In humans, a detailed history, provocation tests, response and localisation of pain on palpation, and detailed 3D imaging allow a robust diagnosis of various back pathologies to be made.
If the question is “can we objectively evaluate back pain?”, the answer, in the author’s opinion, is no. It is hard to argue against an obvious pain response on palpation (see Video), but it must be deciphered whether this is contributing to the presenting problem and primary or secondary to a problem elsewhere, such as hindlimb lameness.
Other cases of back pain may be more subtle in their effect on performance – for example, a decrease in jumping performance, with the horse becoming hollow over jumps. This presents a diagnostic challenge and a secure diagnosis can be difficult to achieve.
To proceed, clinicians would, ideally, have a meaningful set of criteria for the assessment of pain and an objective system of quantifying it. Unlike with lameness, where asymmetry of weightbearing and push-off can be compared to the contralateral limb (alongside other nuances of lameness diagnosis), no such comparison is possible in the back unless symmetry between left hind diagonal weightbearing, and right hind diagonal weightbearing, is compared.
Objective evaluation of back movement
While an objective evaluation of back pain is likely not possible, we can objectively measure back movement.
Optical motion capture – “mocap” – is considered the gold standard to assess 3D movement and orientation of back movement, with a high degree of accuracy. In humans, a similar system has been shown to correctly classify back pain in 97 per cent of patients (Sánchez-Zuriaga et al, 2011). However, in horses, its use is restricted to a gait laboratory and/or treadmill that require habituation and are known to cause kinematic changes (Buchner et al, 1994).
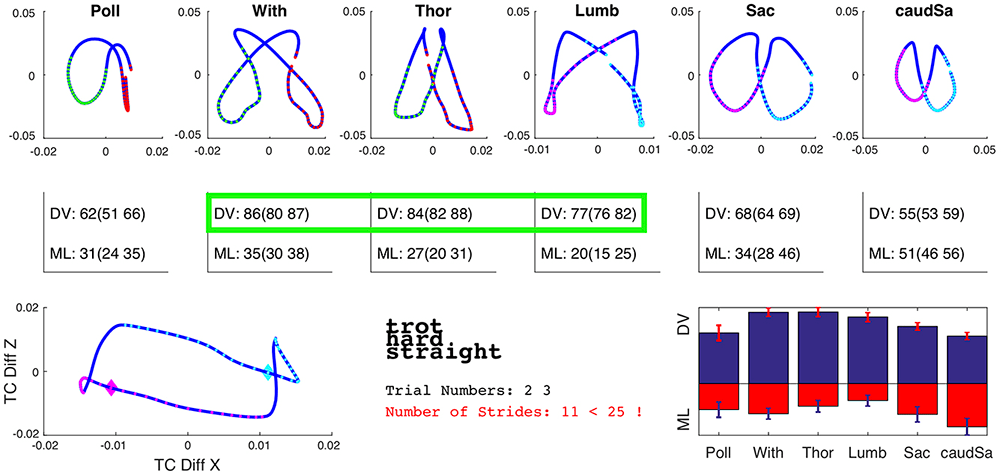
Inertial measurement units (IMUs) have risen to prominence in the diagnosis of lameness. While deemed controversial by some, they undoubtedly provide an objective measurement of movement and can notice asymmetries too small to detect with the human eye. The IMUs are placed along the horse’s spine, allowing back range of motion (ROM) to be measured in three planes – flexion/extension, lateral-lateral ROM and axial rotation (Figure 1). This can be performed in the horse’s normal environment, alongside assessment of lameness (Pfau et al, 2007; Keegan et al, 2002; Church et al, 2009), with concurrent assessment of the horse-rider interaction (Pfau et al, 2009).
When compared to mocap, inertial sensors show acceptable accuracy and good levels of consistency for assessment of back movement (Warner et al, 2010).
Objective data consistently shows a double sinusoidal pattern for dorsoventral movement and a sinusoidal pattern for mediolateral displacement. Dorsoventral movements show greatest amplitude in the caudal thoracic region, and smallest amplitude in the cranial thoracic and sacral regions (Warner et al, 2010). Mediolateral amplitudes tend to decrease between the T6 and L1 vertebrae, before increasing again in the sacral region; however, the relatively small range of motion in the mediolateral direction means asymmetries (lower than 25 per cent) in this plane may be more difficult to detect.
Does this help us? Yes, as we can measure back movement objectively, but what do we compare it to? We are unlikely to have data from before the horse had back pain.
Comparing it to other horses introduces too many variables to make any findings valid. Wennerstrand et al (2004) compared back movement of horses with clinical evidence of back pain with a group of control horses using mocap; data was collected from the horses walking and trotting on a treadmill. In both walk and trot, dorsoventral movement was reduced at the thoracolumbar junction in horses with back pain compared to the control group.
However, one study found if lactic acid was injected unilaterally into the longissimus dorsi muscle, increased extension of the caudal back is noted as part of an overall increased flexion extension (Wennerstrand et al, 2009).
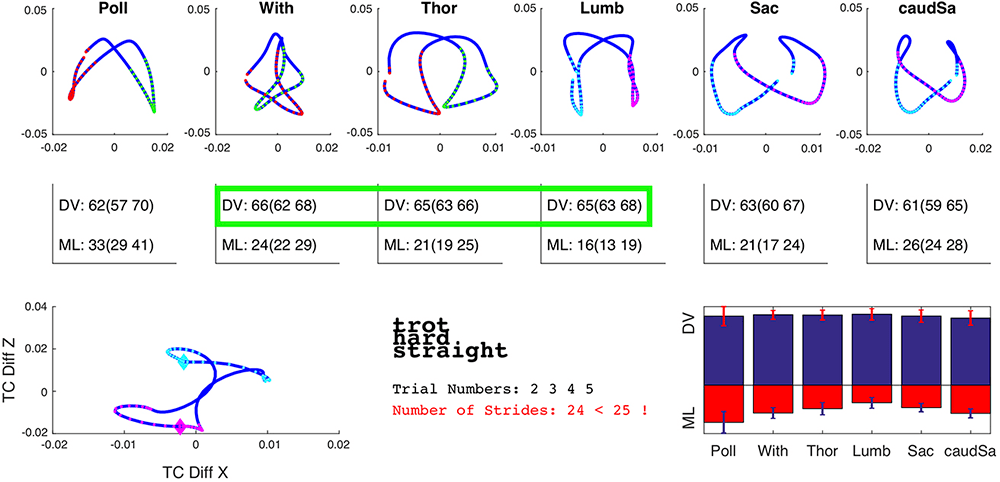
In the author’s clinic, using IMUs, variations in back movement can be objectively measured and compared between horses (Figure 2).
In an attempt to allow the horse to act as its own control, the author is collecting IMU data from horses before and after interspinous ligament desmotomy and subtotal ostectomy. Provided the clinical signs that led to the decision to perform the surgery have resolved, a comparison of back movement before and after the procedure can be performed; preliminary data will be presented at congress.
One would hypothesise elimination of pain would result in “normalisation” of back movement. It may be with collection of sufficient data, a normal range of movement will become evident. Perhaps, more likely, each horse will have a range of movement at each site along the back – especially at the mid-caudal thoracic and cranial lumbar region – and deviation from that normal range would alert attending clinicians to pain returning. This could allow institution of a robust rehabilitation programme before clinical signs advanced.
Effect of lameness on back movement
It is widely accepted resolution of any hindlimb lameness present is imperative prior to focusing on any back pain present.
Horses extend the thoracolumbar spine and decrease the range of motion at the lumbosacral junction in response to hindlimb lameness (Buchner et al, 1996; Gómez Álvarez et al, 2007). This may predispose a horse to the development of impinging spinous processes.
Using IMUs, Greve et al (2017) showed following resolution of naturally occurring hindlimb lameness (with diagnostic analgesia), ROM significantly increased in flexion-extension at T13, axial rotation at T13, T18 and L3, and lateral-lateral ROM at L3. It can, therefore, be concluded hindlimb lameness causes asymmetry of back movement. This is likely, in the chronic state, to cause changes in back dimensions (associated with epaxial muscle atrophy) and altered functionality. Whether similar changes in back movement are seen in horses with primary back pain and no associated lameness has yet to be determined.
If lameness is not present or has resolved, measurable asymmetric movement may suggest presence of back pain. The problem with interpretating this data is making the link between “active”, relevant back pain and asymmetric movement or reduced ROM. Collection of IMU data of back movement before and after surgery – provided clinical signs of back pain have resolved – will allow assessment of whether back pain is linked to asymmetric movement.
Objective evaluation of epaxial (multifidus) muscle mass
Lame horses have a reduced ability to increase epaxial muscles mass – suggesting lameness causes a reduction in the ability to use the thoracolumbar epaxial muscles normally (Greve and Dyson, 2015).
Lower back pain in humans causes inhibition of deep spinal stabilising muscles adjacent to injury affecting multifidus and transverse abdominis muscles (Barker et al, 2004). Saddle slip to one side occurs in approximately half of horses with hindlimb lameness (Greve and Dyson, 2013; 2014). This may be the result of asymmetric thoracolumbar movement and/or asymmetric epaxial muscling.
In Thoroughbred racehorses, osseous spinal pathology has been shown to cause an ultrasonographically measurable left/right asymmetry in multifidus at, or close to, the level of pathology (Stubbs et al, 2010). The same has been demonstrated in humans, with atrophy of multifidus seen ipsilateral to symptoms in patients with acute, or subacute, lower back pain (Hides et al, 1994); this is measured on MRI scans and occurs within days of the onset of back pain.
It has also been shown these muscles require specific therapeutic exercises to be activated after initial injury has been treated. If this is addressed, a reduction in the one-year injury recurrence rate – from 84 per cent to 30 per cent – has been demonstrated (Hides et al, 2001).
Stubbs et al (2011) demonstrated, by implementing dynamic stabilisation exercises, hypertrophy of multifidus could be achieved.
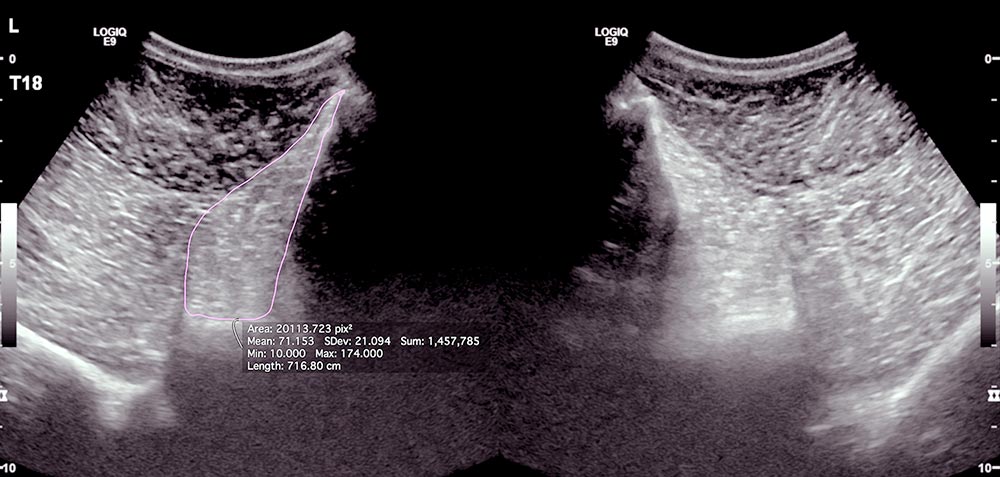
We are unable to perform MRI of horses’ backs, but ultrasound examination and subsequent measurement of the cross-sectional area of multifidus muscle is possible. While an atrophied multifidus muscle does not necessarily cause back pain, it seems clear it provides strong evidence of back pain.
If the same is true in horses, as is seen in humans, the site of asymmetry could indicate the site of pathology and, therefore, provide an objective – yet indirect – measurement of back pain. Unfortunately, it will not be possible to ascertain when the pathology occurred and, as aforementioned, in humans, the multifidus muscle stays atrophied unless specifically targeted.
The most commonly implicated pathology in horses with back pain is impinging spinous processes – and it is certainly the easiest to demonstrate with conventional radiography. The most frequently affected spinous processes are those in the mid to caudal thoracic region (Walmsley et al, 2002).
The author has an ongoing study collecting data on the multifidus muscle cross-sectional area in horses with pain associated with impinging spinous processes (Figure 3). The preliminary data will be presented at congress.
Summary
While a number of clinical features exist for equine practioners to subjectively evaluate the sore backed horse, this article has outlined some advances in assessing back movement and pain objectively.
We still have a lot to learn about the significance of such measurements, but with collection of data – and linking this to demonstrable pain and pathology – we hope to establish this in the near future.
Acknowledgements
The author would like to thank Thilo Pfau and Charlotte Power-Kane for their input.
