15 May 2017
Andy Durham explores the types and diagnostic techniques for this common concern, and its contribution to airway disease.

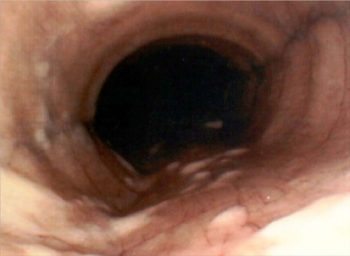
Figure 2a. Small mucus blobs may be seen in normal horses.
Respiratory disease is the fourth most common equine health concern reported by horse owners1, with signs including cough, nasal discharge (Figure 1) and abnormal breathing patterns.
Airway diseases
Interstitial diseases
Pleural diseases
Cardiovascular diseases
Extrathoracic diseases
However, the existence of subclinical disease should not be underestimated in performance animals that frequently demonstrate no adverse effects other than limitation of their exercise capacity.
Respiratory diseases may be divided into those affecting the airways, the pulmonary interstitium and/or the pleura, with a few other non-respiratory diseases that may appear clinically similar (Panel 1).
Airway diseases are by far the most-common type of respiratory disease in horses, with recurrent airway obstruction (RAO, also known as severe equine asthma2) by far the most commonly diagnosed problem.
Despite the characteristically marked airway neutrophilia in RAO cases, infection plays no direct role in the aetiology of the condition. Infectious agents are, however, an important cause of airway diseases – especially in young horses both in a direct and straightforward fashion, and in a more complex and nebulous fashion in the causation of so-called inflammatory airway disease (also known as mild to moderate equine asthma2).
Differentiation and diagnosis of these conditions depends on examination of both the horse and its environment, as well as appropriate diagnostic techniques and samples.
Further to a standard clinical examination including auscultation, further commonly used diagnostic techniques relevant to suspected respiratory disease include measurement of systemic inflammatory biomarkers, serology, nasal/nasopharyngeal/guttural pouch microbiology, auscultation with a rebreathing bag, radiography, ultrasonography, endoscopic examination of the airway and collection of samples of respiratory secretions (Figure 2).
Commonly, a specific diagnosis requires multiple diagnostic techniques, and careful selection and interpretation of test results is crucial, given the frequent close similarity of individual test results between disparate clinical (and subclinical) conditions.
For example, both infectious and non-infectious airway diseases may present similarly in an individual horse (for example, cough and poor performance) and may demonstrate similar tracheal cytology (for example, moderate neutrophilia).
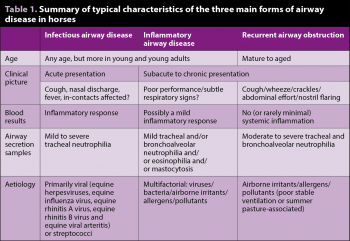
However, historical factors such as age, environment and in-contact morbidity – along with systemic inflammatory biomarkers, serology and bacteriology – may aid in distinguishing the conditions2 (Table 1).
Although a relatively high prevalence of influenza vaccination exists in active competition horses, younger horses may not always be vaccinated and are known to suffer from “immunity gaps” during their standard primary course of vaccinations3.
Furthermore, three of the four licensed equine influenza vaccines in the UK do not contain representatives of circulating equine influenza strains (Richmond ’07-like, Florida clade 2 strains) as recommended by the World Organisation for Animal Health expert surveillance panel, leading to suboptimal protection of the “national herd”4.
Equine viral arteritis (EVA) infection is very rare in the UK and has not been reported since 2012, but remains a notifiable disease in stallions or mares that have been mated or inseminated within the previous 14 days.
Additional viruses have also been implicated in respiratory disease in horses either directly and/or as contributors to inflammatory airway disease, although their relevance remains equivocal in many instances. These primarily include gamma-herpesviruses (EHV-2 and 5) and equine picornaviruses (equine rhinitis A and B viruses).
Probably the only unequivocal bacterial respiratory pathogen of UK horses is Streptococcus equi subspecies equi, although tracheal infection with other beta-haemolytic streptococci (for example, Streptococcus equi subspecies zooepidemicus), alpha-haemolytic streptococci (Streptococcus pneumoniae), Actinobacillus species, Haemophilus species and Pasteurella species may also contribute to airway disease. Very rarely, cases of parasitic airway disease (Dictyocaulus species) may also be encountered.
Further to clinical examination and assessment of the horse’s history and environment, the major diagnostic techniques relevant to identification of infectious disease include serum testing for inflammatory markers, and serology and sampling from the respiratory tract for isolation of potential pathogens.
Haematology is probably the most basic clinicopathologic means of inferring a systemic inflammatory response, but is neither very sensitive nor specific. Neutrophilia is the most-common haematologic indicator of inflammation, although is also seen during a stress response (in addition to lymphopenia). Even when neutrophilia genuinely indicates inflammation, this does not necessarily equate with infection as non-infectious inflammatory stimuli, such as tissue damage, may also cause neutrophilia. Conversely, neutropenia may also reflect inflammation either as a result of consumption or sequestration.
Some basis exists in this dogma, although it is variable and inconsistent. In one study of EHV-1 respiratory infection, the group average haematologic pattern began with neutrophilia on day one with a reducing neutrophil to lymphocyte ratio over the first five days. However, on an individual basis,only 40% of horses demonstrated greater lymphocyte than neutrophilnumbers (“reversed differential”) duringthe investigation5.
For many years now, the major acute phase proteins used in equine clinical pathology have been serum amyloid A (SAA) and fibrinogen. Clinical value of SAA is firmly established as a sensitive, specific and fast reacting acute phase protein. Fibrinogen also continues to be used widely, despite clear inferiority in both technical and diagnostic respects.
Iron is an essential element for many microbial metabolic processes, including their pathogenicity. Host immunity has evolved to reduce iron availability in the serum as a defensive response during acute inflammation.
This is mediated by the acute phase protein hepcidin and other cytokines that reduce iron absorption from the intestine and rapidly internalise circulating iron within macrophages, where it is unavailable for the potential bacterial or viral invader6. Thus, inflammation is characterised by hypoferraemia.
Following several supportive clinical and experimental studies7-10, the Liphook Equine Hospital Laboratory became the first clinical diagnostic laboratory to offer serum iron as an inflammatory marker in horses. Its diagnostic superiority compared with fibrinogen is well established7,11,12 and subsequent clinical use has been equally impressive.
A study of endotoxaemia-induced inflammation in horses showed low serum iron to be detected by 16 hours following onset of inflammation compared with 8 hours for SAA and 24 hours for fibrinogen13.
Several serologic assays may be used to support the involvement of viral agents, although it should be remembered serology is always a weak means of diagnosing any infectious disease, as positive results reflect prior exposure (or even vaccination) and not necessarily current clinical infection.
Given the relatively high vaccination prevalence against equine influenza, serology is not often used diagnostically and only generally employed in unvaccinated individuals. Alpha-herpesviruses are undoubtedly common respiratory pathogens in young horses; although, interestingly, seropositivity against EHV-1 or 4 is seen in fewer than 1% of submitted blood samples in the UK14.
In contrast, more than 500 horses were tested as seropositive for EVA in the UK in2016 (4.6% of submitted samples)14, although, as aforementioned, this reflected previous exposure (presumably abroad)rather than current infection, with no risk toin-contact horses.
Equine picornaviruses may cause clinical respiratory disease affecting both upper and lower airways15, or contribute to inflammatory airway disease.
However, exposure in clinically normal horses is also common. Strangely, despite the suggestion equine picornaviruses are common equine pathogens, only a single case of a seropositive horse was seen from more than 600 submitted samples in 2016 in the UK14, perhaps suggesting assay insensitivity.
Methods aiming to isolate the implicated pathogen and/or its genetic material by PCR are the preferred means of diagnosing infection, although it should be remembered some infectious agents can be isolated from clinically normal horses.
Viruses such as equine influenza, EHVs, equine arteritis virus and picornaviruses may be isolated from nasal swabs; although, as aforementioned, the presence of gamma-herpesviruses and picornaviruses in particular may be incidental in many cases.
Bacterial isolation is also not straightforward. S equi subspecies equi is generally regarded as pathologically relevant when isolated from any site. Although not yet a popular technique in the UK, nasal washes are the preferred diagnostic sample in suspected acute strangles cases as a greater surface area of nasopharyngeal mucosa is sampled16. Guttural pouch lavage is only applicable in subacute to chronic cases and is for the purpose of identifying potential chronic carriers of disease.
Few, if any, other bacterial species are relevant when isolated from nasal or nasopharyngeal swabs in horses with suspected respiratory disease, as a wide variety of bacterial species may exist in the nasal passages of normal horses.
In contrast, other streptococci, Pasteurella, Haemophilus and Actinobacillus may be relevant when isolated from the airway of a horse with cytologic evidence of airway inflammation and when steps have been taken to avoid contamination of the collected sample with nasopharyngeal bacteria.
Isolation of these bacteria from an airway with no abnormal mucus accumulation, less than 20% neutrophils or with evidence of squamous epithelial cells (derived from the pharynx) should not be regarded with pathologic relevance.
Isolation of other bacterial species from the equine trachea – such as Enterobacteriaceae, staphylococci, enterococci and so on – is rarely of any pathologic relevance and does not merit antimicrobial therapy, except in cases of clinical bronchopneumonia.
Pseudomonas species are a common isolate from samples collected from the equine respiratory tract, but are generally associated with infection within wet channels of collection equipment rather than from the horse itself.
Finally, Dictyocaulus arnfieldi is a rarely encountered tracheobronchial nematode infecting some horses – especially with donkey contact. The author has never yet encountered a case in 29 years in equine practice, although at least 14 cases were seen in the UK last year14.
Infectious respiratory disease in young horses is probably not uncommon, although diagnostic data would suggest it is suspected more commonly than it actually occurs.
One should never underestimate the importance of good air hygiene in respiratory disease of horses of any age, as airborne irritants and pollutants are known to play a role in many cases seen in practice.