25 Jun 2018
Alison Bennell and Jodie Hughes discuss the importance of familiarity with drugs and protocols, and good organisation and forward planning, in a stress-free anaesthesia in the field.

Figure 1. Plasma concentration with a "top-up" technique.
The first part of this article, “Pros and cons of adopting TIVA techniques for field anaesthesia” (VT48.14), discussed the advantages and disadvantages of total IV anaesthesia (TIVA) and some of the practical aspects, such as equipment and location.
This article will focus on the available methods of performing TIVA safely in a field situation.
Careful consideration of the planned procedure is vital, as TIVA should not be used for longer than a period of one hour. Beyond this period, drug accumulation will occur, causing protracted and undesirable recoveries.
Could the planned procedure take longer than one hour? Are complications that could significantly increase the duration of anaesthesia required highly likely? If the answer is yes to either question, an inhalational anaesthetic regimen should be considered in a hospital situation.
It can be challenging to perform field anaesthesia and surgery alone, especially if the surgery is more involved than a routine castration, for example. In an ideal world, two veterinary surgeons would be present – one to focus on the anaesthesia and one on the surgery. If this is not feasible, an RVN or competent veterinary student will be able to monitor the anaesthetic and administer top-ups, as necessary, under guidance.
A very sensible owner may be able to administer top-up doses, but this can be daunting for many owners and may distract attention from the surgical procedure.
A full history should be obtained, including tetanus vaccination status, and a full preanaesthetic clinical examination should be performed to rule out any comorbidities that may impact on anaesthesia. The presence of abnormal clinical findings – such as pyrexia, arrhythmias, respiratory disease or poor body condition – would be an indicator to perform further diagnostics and delay elective surgery. The surgical site should also be assessed – for example, to ensure two testes are present prior to castration.
An estimation of the horse’s bodyweight should be made, as discussed in part one of this article. The owner should be counselled in what to expect from anaesthesia, informed consent gained and section IX of the passport checked to ascertain whether the animal is destined for human consumption, as this will influence drug choice.
Withholding feed prior to anaesthesia in horses is controversial. The aim of withholding food is to reduce the amount of ingesta within the gastrointestinal tract, which may inhibit ventilation when the horse is recumbent. However, very little evidence supports this theory. It is probably sensible to avoid concentrate feed in the six hours prior to anaesthesia, but access to forage/normal grazing will aim to keep animals calm and in their normal routine prior to anaesthesia.
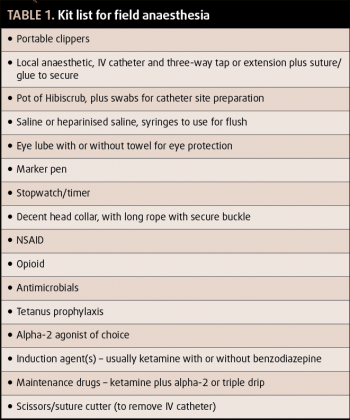
Equipment should be fully prepared prior to inducing anaesthesia; a basic kit list is outlined in Table 1. In an ideal world, equipment for endotracheal intubation and oxygen supplementation would be available, but this is often not practical in a field situation, and may distract from monitoring and maintenance of anaesthesia.
If an endotracheal intubation is to be performed, thorough lavage of the mouth is indicated to minimise the risk of aspiration. Secure venous access should be gained with a 14 or 16-gauge catheter in the jugular vein. Performing “off-the-needle” anaesthesia is never recommended, as it is dangerous and difficult to administer top-up anaesthetic agents to a moving horse, and consciousness can be regained rapidly.
If a single vet is to perform the surgery and anaesthesia alone, a long extension line can be attached to the catheter to allow drug administration from a distance. Emergency top-up doses of the chosen anaesthetic agent should be pre-drawn, clearly labelled and readily accessible in case of movement under anaesthesia. Do not place these top-ups in a location where they may become inaccessible due to horse movement.
The horse’s temperament will somewhat dictate the dose and route of administration of sedative agents. It is the authors’ preference, where the horse is amenable, to administer sedative agents IV after the jugular catheter has been placed.
Acepromazine is commonly recommended for administration prior to general anaesthesia, as it has been associated with a reduced risk of perioperative mortality¹. However, the onset of acepromazine is 40 minutes, which is impractical in most field situations. It is sensible to administer sedatives at the location selected for the surgery, as moving sedated horses can be tricky and unsafe.
Alpha-2 adrenoreceptor agonists are the mainstay of equine sedation, with xylazine, detomidine and romifidine being licensed for this purpose in horses. All three are suitable for sedation prior to induction of general anaesthesia. Romifidine is associated with less ataxia, and can, therefore, provide a more stable base for induction of anaesthesia.
A profound level of sedation should be obtained prior to induction, which is indicated by the head dropping to a lowered height and the lack of response to stimuli, such as clapping or a gentle tap on the nose.
All horses undergoing a surgical procedure require analgesia to be provided. Additional analgesia should be provided at this stage, with NSAIDs and opioid administration being routine, dependent on the procedure being performed. The choice of licensed NSAID depends on ease of administration, and the plan for postoperative analgesia provision.
No evidence is available to suggest one NSAID is better than another for provision of analgesia. The opioid should be administered after the sedative agents to avoid unwanted excitatory side effects, such as walking or head twitching. The most common opioids used in equine practice are morphine, buprenorphine and butorphanol. Selection will depend on the level of pain expected and legal obligations.
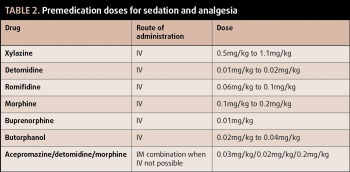
Suitable preanaesthetic doses for sedatives and opioids can be found in Table 2. It is worth considering a full mu agonist (for example, morphine) will provide superior analgesia in comparison to other opioids. When butorphanol is administered at analgesic doses, it can cause locomotor excitation, which can lead to difficult inductions.
Do not forget to prepare a local anaesthetic agent to administer once anaesthesia has been induced. This is the only method of providing complete prevention of pain transmission and will significantly improve the stability of your anaesthetic plane. Commonly used local anaesthetic agents include mepivacaine, lidocaine and bupivacaine. Selection should be based on the required duration of action and speed of onset.
The two commonly used anaesthetic induction agents in horses are ketamine and thiopental. Ketamine is a dissociative anaesthetic agent readily available in the UK. Its main benefits are relative cardiorespiratory stability in healthy horses and excellent analgesia.
The cranial nerve reflexes are maintained, which can make it difficult to assess the depth of anaesthesia. However, these reflexes are uncoordinated and not protective, so care must still be taken to protect the eyes. Ketamine does not provide muscle relaxation and is, therefore, usually combined with a benzodiazepine or guaifenesin.
If guaifenesin is used as part of the anaesthesia maintenance protocol, it should be avoided at induction to reduce the risk of overdose. Ketamine has a relatively slow onset of one to two minutes, allowing for a calm preparation for induction. This fact, though, should be remembered when topping up anaesthesia.
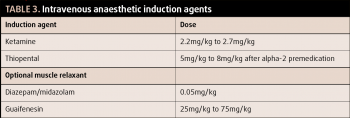
Thiopental is no longer produced in the UK with a veterinary market authorisation. It is, therefore, not commonly available to general practitioners. It has a rapid onset of 30 seconds, causing an abrupt induction of anaesthesia. Thiopental cannot be administered as an infusion or by top-up boluses to maintain anaesthesia. Anaesthetic induction doses are presented in Table 3.
For induction, the horse should be standing squarely, with one or two trained personnel holding a head collar rope. If two people are present, one should stand either side of the horse. The head should be restrained in a neutral position.
Once the horse starts to become recumbent, handlers should apply pressure to the shoulder, guiding the horse backwards in a straight line. The aim is for the horse to gain sternal recumbency and then be rolled into the selected recumbency in a gentle manner, avoiding trauma from hitting the ground hard. Handlers must be aware of their surroundings at this stage and able to move quickly as necessary.
Once recumbent, the lower forelimb should be extended cranially and the eyes protected by being covered with a towel. The head collar buckles should be padded to prevent facial nerve damage.
Assessing anaesthetic depth with IV techniques can be challenging. Cranial nerve reflexes are maintained with ketamine-based anaesthesia, making the palpebral reflex, eye position and lachrymation unhelpful for monitoring depth. Ketamine will also cause horizontal nystagmus.
Horses under too light a plane of anaesthesia may show fast horizontal nystagmus, spontaneous swallowing, changes in respiratory pattern and limb or neck movement. If, at any point, the depth of anaesthesia is considered too light, a bolus of ketamine or thiopental should be administered immediately.
The cardiovascular system should be monitored by pulse palpation and mucous membrane assessment. Respiratory rate and pattern should also be observed. It is possible to use portable pulse oximeters, but they can be inaccurate due to the vasoconstriction and bradyarrhythmias induced by alpha-2 agonists.
Two well-reported methods for performing TIVA exist: bolus and infusion.
This method involves intermittent bolus administration of the anaesthetic agents to maintain anaesthesia. This leads to a changing plasma concentration of the drug, with peaks and troughs.
Immediately following a bolus, the plane of anaesthesia will be deep, and immediately prior to a bolus, the plane will be light (Figure 1). The benefits of this technique include simplicity and, as large volumes of drugs are not prepared (which may be left unused), it is often inexpensive.
Anaesthesia is usually maintained with a combination of ketamine and an alpha-2 adrenoreceptor agonist. A third of the induction dose of ketamine is administered after 10 minutes of anaesthesia, followed by a quarter to a third of the induction dose every 10 minutes thereafter, until the end of the procedure.
Repeat boluses of the alpha-2 adrenoreceptor agonist are administered to maintain the plane of anaesthesia and muscle relaxation. The frequency depends on the selected drug. The short duration of action of xylazine makes it the most appropriate choice, and standard protocol is to administer a third of the premedication dose with every second ketamine top-up.
The combination of several agents (as a “triple drip technique”) delivered as an infusion to maintain anaesthesia following induction (Figure 2). Delivery of the drugs by this method allows maintenance of a stable plasma concentration. It is often described as a continuous rate infusion; however, the rate may be altered to achieve the desired plane of anaesthesia. Top-up boluses of ketamine or thiopental should still be available in case of sudden movement.
Commonly, a bottle of guaifenesin is spiked with ketamine and an alpha-2 adrenoreceptor agonist. It is then delivered via an IV giving set. The authors’ favoured “recipe” is 1g ketamine and 0.5g xylazine added to 500ml of 10% guaifenesin. This is initially delivered at 1ml/kg/hour (approximately three drops/second for a 500kg horse with a standard giving set) and titrated to effect.
It is usual to start the infusion at a fast drip for approximately five minutes, then decrease the rate for the following 30 minutes to three drops/second, for a 500kg horse. After this, the rate can be decreased further to maintain unconsciousness, while aiming to achieve a good recovery from anaesthesia.
Monitoring horses under triple drip anaesthesia can take practice, as many appear “light”. Signs of overdose are paradoxical and can include extensor rigidity, and can be interpreted as under-dosing.
Guaifenesin is an irritant to the vascular endothelium. The catheter must be flushed thoroughly with saline prior to removal. The total dose of guaifenesin should not exceed 100mg/kg.
Recovery from injectable anaesthesia is generally of better quality than recovery from inhalational anaesthesia (see video at vettimes.co.uk). External stimuli should be kept to a minimum to prevent a premature attempt to stand.
Observers should be kept at a safe distance and everyone should have a clear exit route. Gentle restraint can be applied by holding a long rope attached to a secure head collar, which will allow head control when recovering and once standing. In smaller horses, a second handler can hold the tail on recovery, but this is not recommended for anything larger than a small pony.
Initially, the horse may be ataxic and should not be encouraged to walk to the stable until more steady. Recovery to standing usually takes 10 to 30 minutes. Once the horse is standing and fully recovered, the IV catheter can be removed and the owner given clear postoperative and postanaesthetic instructions.